Chromosome Condensation Facilitates Chromosome Segregation
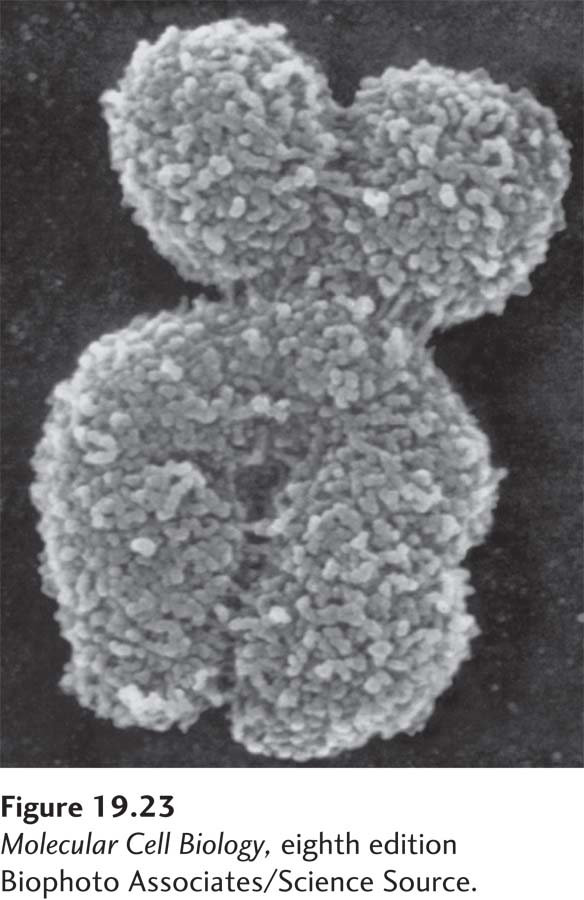
FIGURE 19-23 Scanning electron micrograph of a metaphase chromosome. During metaphase, the chromosomes are fully condensed, and the two individual sister chromatids are visible.
[Biophoto Associates/Science Source.]
Chromosome segregation not only requires the building of the apparatus that segregates chromosomes, but also requires that the DNA be compacted into travel-friendly structures. Any attempt to segregate the long and intertwined DNA-protein complexes present in interphase cells would lead to breakage of the DNA and hence loss of genetic material. To avoid this fate, cells compact their chromosomes during prophase into the dense structures we have become acquainted with through light or electron microscopy (Figure 19-23).
Chromosome condensation results in a dramatic reduction in chromosome length, up to 10,000-fold in vertebrates. The second key aspect of the compaction process is the untangling of the intertwined sister chromatids. This process, called sister chromatid resolution, is mediated in part by the decatenation activity of topoisomerase II and goes hand in hand with the condensation process.
Recent studies have shown that chromosome compaction occurs via the generation of consecutive loops that lead to the formation of a fiber at the bases of the loops (Figure 19-24a). These fibers are then compressed, leading to further chromosome compaction (Figure 19-24b). Central to the process of chromosome condensation is a protein complex known as condensin. This protein complex, which is related to the cohesins that link sister chromatids together after DNA replication, was first identified based on its ability to promote chromosome condensation in frog extracts. Like cohesins, condensins are composed of two large coiled-coil SMC protein subunits that associate through their ATPase domains with non-SMC subunits. When condensin function is lost in cells, chromosomes do not condense and sister chromatid tangles are not resolved. Condensins are likely to create the intrachromosomal linkages that package chromosomes into consecutive loops. Their association with chromosomes is facilitated by mitotic CDKs and Aurora B. These two protein kinases phosphorylate histone H2A, allowing condensins to bind chromatin.
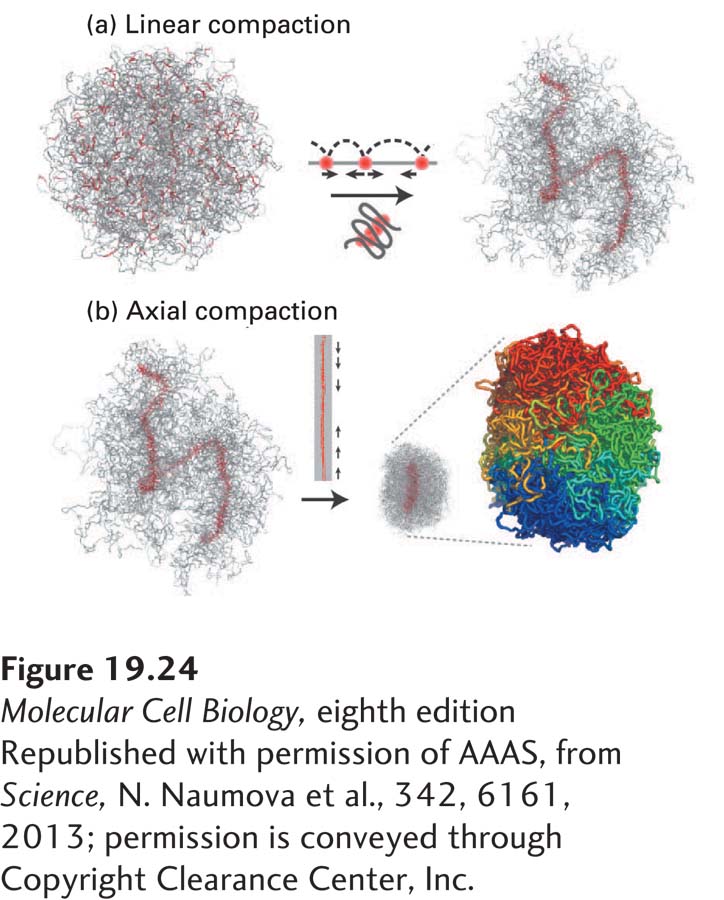
Figure 19-24 A model for chromosome compaction during mitosis. (a) The folding of chromosomes into consecutive loops leads to the formation of proteinaceous fibers at loop bases. (b) These chromosome axes are then further compressed to generate highly condensed chromosomes. The proteins responsible for loop formation are likely to be condensins.
[Republished with permission of AAAS, from Science, N. Naumova et al., 342, 6161, 2013; permission is conveyed through Copyright Clearance Center, Inc.]
Finally, dissociation of cohesins leads to further compaction of chromosomes. A large fraction of cohesins are removed from chromosomes during prophase (see Figure 19-17, step 3). This process is mediated by phosphorylation of cohesins by Polo kinase and Aurora B kinase. In most organisms, cohesins are maintained only around centromeres. Cohesins around centromeres are protected from phosphorylation-dependent removal by protein phosphatase 2A (PP2A). This phosphatase is recruited to centromeric regions by a member of a family of PP2A targeting factors known as the Mei-S332/Shugoshin family of proteins. The protected pool of cohesins provides the resistance to the pulling force exerted by microtubules necessary to establish tension at bi-oriented kinetochores. As we will see in Section 19.8, this protection mechanism also plays an essential role in establishing the meiotic chromosome segregation pattern.

