The Spindle Assembly Checkpoint Pathway Prevents Chromosome Segregation Until Chromosomes Are Accurately Attached to the Mitotic Spindle
The spindle assembly checkpoint pathway prevents entry into anaphase until every kinetochore of every chromatid is properly attached to spindle microtubules. If even a single kinetochore is unattached or not under tension, anaphase is inhibited, as such a defect would almost certainly lead to chromosome loss if mitosis ensued in its presence. To achieve this, cells harbor a surveillance mechanism that prevents anaphase entry in the presence of tensionless or unattached kinetochores. The spindle assembly checkpoint pathway recognizes unattached kinetochores. However, as we saw in Section 19.5, kinetochores of sister chromatids often attach to microtubules emanating from the same pole (syntelic attachment), or a single kinetochore attaches to microtubules that originate from two different poles (merotelic attachment). How are such faulty attachments recognized? Microtubule-kinetochore interactions that are under insufficient tension are destabilized by Aurora B phosphorylation of the microtubule-binding component of the kinetochore, the Ndc80 complex. This leads to the generation of unattached kinetochores, which are then recognized by the spindle assembly checkpoint pathway. In this manner, Aurora B and the spindle assembly checkpoint pathway collaborate during every cell cycle to accurately attach every single pair of sister chromatids to the mitotic spindle in the correct, bi-oriented manner.
Components of the spindle assembly checkpoint pathway bind to unoccupied microtubule binding sites at kinetochores and create an anaphase inhibitory signal that ultimately inhibits APC/CCdc20. Recall that APC/CCdc20-mediated ubiquitinylation of securin and its subsequent degradation are required for the activation of separase and entry into anaphase (see Figure 19-25). When a kinetochore is not attached to microtubules, the outer kinetochore component Knl1 is phosphorylated by the spindle assembly checkpoint pathway kinase Mps1 (Figure 19-31). This phosphorylation, in turn, recruits other checkpoint pathway components to the unattached kinetochore. Critical for the shutting down of APC/CCdc20 is the recruitment and activation of the Mad1-Mad2 complex by unattached kinetochores. Importantly, the activated Mad1-Mad2 complex can convert inactive Mad2 molecules in the cytoplasm to an active conformation that is capable of binding to and inhibiting APC/CCdc20. Mad2 bound to APC/CCdc20 recruits additional checkpoint factors to the complex to form the mitotic checkpoint complex (MCC). The MCC then prevents APC/CCdc20 from recognizing and ubiquitinylating substrates. This elegant model for the spindle assembly checkpoint pathway can account for the ability of a single unattached kinetochore to inhibit all the cellular Cdc20 until the kinetochore becomes properly associated with spindle microtubules.
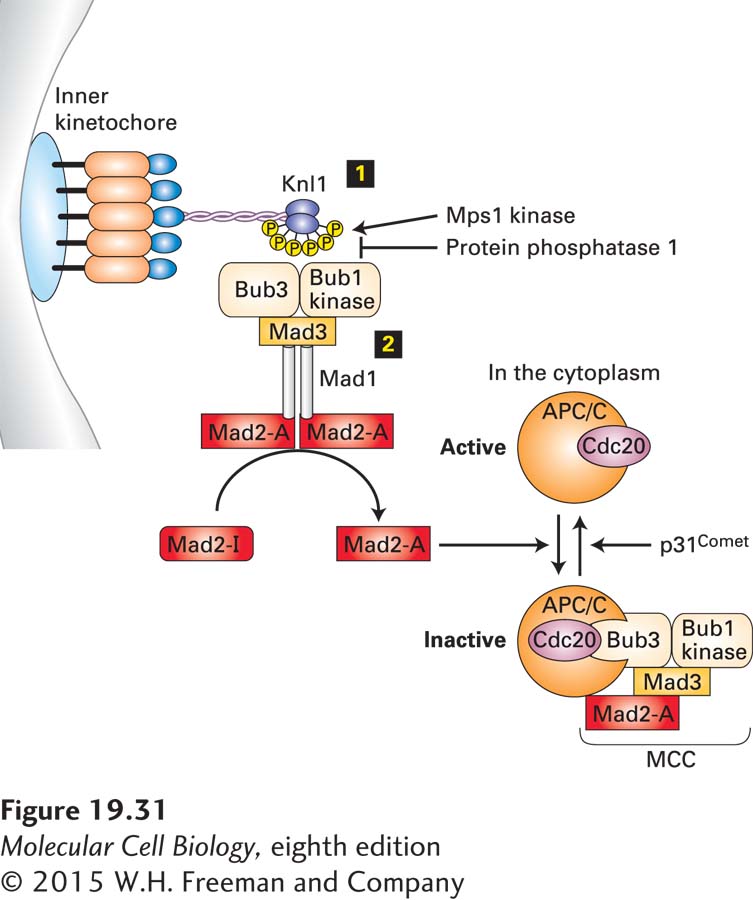
FIGURE 19-31 The spindle assembly checkpoint pathway. The spindle assembly checkpoint pathway is active until every single kinetochore has attached properly to spindle microtubules. When a kinetochore is unattached, the outer kinetochore component Knl1 is phosphorylated by the checkpoint kinase Mps1 step 1. Phosphorylated Knl1 then binds the checkpoint kinase Bub1-Bub3 and the checkpoint protein Mad3. These three proteins, in turn, recruit the Mad1-Mad2 complex to the kinetochore. The Mad1-Mad2 complex bound to a kinetochore is in the active form (shown as Mad2-A; step 2 ) and has the ability to convert inactive Mad2 (Mad2-I) in the cytoplasm into active Mad2 that is able to bind APC/CCdc20 and inhibit it. Complete inhibition of APC/CCdc20 requires the recruitment of the checkpoint factors Bub1-Bub3 and Mad3 into the complex. Together, these proteins form the mitotic checkpoint complex (MCC) that prevents APC/CCdc20 from recognizing and ubiquitinylating its substrates. Silencing of the spindle assembly checkpoint pathway occurs once all kinetochores have attached to microtubules in a tension-generating manner. Protein phosphatase 1 then dephosphorylates Knl1, thereby eliminating checkpoint protein binding sites at kinetochores. In addition, p31comet disassembles the MCC. See E. Foley and T. Kapoor, 2013, Nature Rev. Mol. Cell Biol. 14:25.
Once all chromosomes have attached to the mitotic spindle in the correct amphitelic manner, the spindle assembly checkpoint pathway must be silenced to allow APC/CCdc20 to degrade securin and initiate anaphase. Silencing of the spindle assembly checkpoint pathway occurs through multiple mechanisms. Protein phosphatase 1 dephosphorylates Knl1, thereby eliminating checkpoint protein binding sites at kinetochores. In addition, a protein known as p31comet disassembles the MCC, thereby activating APC/CCdc20 (see Figure 19-31).
The spindle assembly checkpoint pathway is essential for viability in mice, highlighting the importance of this quality-control pathway during every cell division. If anaphase is initiated before both kinetochores of a replicated chromosome become attached to microtubules from opposite spindle poles, chromosomes are mis-segregated in a process called nondisjunction. The resulting condition, in which cells have either lost or gained whole chromosomes, is called aneuploidy. Aneuploidy has profound effects on human health and fitness. It leads to the misregulation of genes and can contribute to the development of cancer. When nondisjunction occurs during the meiotic division that generates a human egg or sperm, trisomy (gain of a chromosome) or monosomy (loss of a chromosome) can be the result. As we will see in Section 19.8, meiosis is especially prone to nondisjunction, which can lead to spontaneous abortions or Down syndrome.
