Recombination and a Meiosis-Specific Cohesin Subunit Are Necessary for the Specialized Chromosome Segregation in Meiosis I
As we have seen, in metaphase of meiosis I, both sister chromatids in one (replicated) chromosome associate with microtubules emanating from the same spindle pole, rather than from opposite poles as they do in mitosis (see Figure 19-36). Two physical links between homologous chromosomes resist the pulling force of the spindle until anaphase: (1) the chiasmata that result from crossing over between chromatids, and (b) cohesins distal to the crossover point (see Figure 19-37b, top). Evidence for the linking function of recombination during meiosis comes from the observation that when recombination is blocked by mutations in proteins essential for the process, chromosomes segregate randomly during meiosis I; that is, homologous chromosomes do not necessarily segregate to opposite spindle poles.
At the onset of meiotic anaphase I, cohesins between chromosome arms are cleaved by separase. This cleavage is required for homologous chromosomes to segregate. If cohesins were not lost from chromosome arms, the recombined sister chromatids would be torn apart during anaphase I. The maintenance of centromeric cohesion during meiosis I is necessary for the proper segregation of sister chromatids during meiosis II.
916
Studies in many organisms have shown that a specialized cohesin subunit, Rec8, is necessary for the stepwise loss of cohesins from chromosomes during meiosis. Expressed only during meiosis, Rec8 is homologous to Scc1, the cohesin subunit that closes the cohesin ring in the cohesin complex of mitotic cells (see Figure 19-25). Immunolocalization experiments revealed that during early anaphase of meiosis I, Rec8 is lost from chromosome arms but is retained at centromeres. However, during early anaphase of meiosis II, centromeric Rec8 is cleaved by separase, so the sister chromatids can segregate, as they do in mitosis (see Figure 19-37b, bottom). Consequently, understanding the regulation of Rec8-
The mechanism that protects Rec8 from cleavage at centromeres during meiosis I is similar to the mechanism that protects Scc1 at centromeres during mitosis. Recall that during mitotic prophase, protein kinases, chief among them Polo kinase, phosphorylate cohesins in the chromatid arms, causing them to dissociate and eliminating cohesion on chromatid arms by metaphase. However, cohesion at the centromeres is maintained because a specific isoform of protein phosphatase 2A (PP2A) is localized to centromeric chromatin by members of a family of proteins known as Mei-
Cohesin removal differs for meiosis I because when Rec8 replaces Scc1 in the cohesin complex, the complex does not dissociate in prophase when it is phosphorylated. The meiotic cohesin complex can be removed from chromatin only by the action of separase. Rec8 also differs from Scc1 in that it must be phosphorylated by several protein kinases to be cleaved by separase. During meiosis I, the centromere-
Meiosis I is much more error-
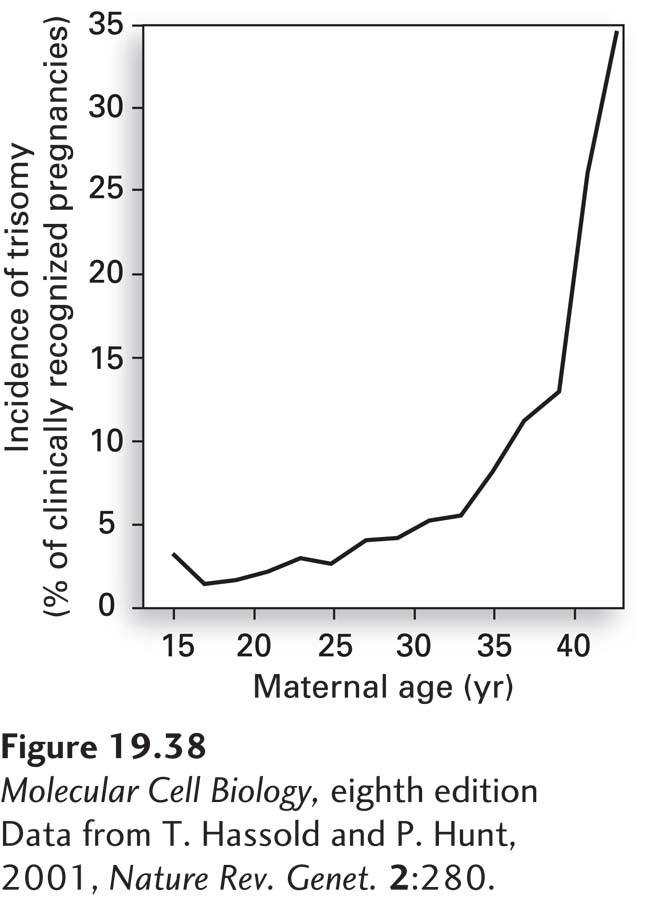
Nondisjunction in meiosis I increases dramatically with maternal age. Whereas fewer than 0.1 percent of babies born to women under the age of 30 have Down syndrome, this rate increases to 3.5 percent at age 45. This increase in chromosome nondisjunction with maternal age is not specific to chromosome 21. The incidence of trisomy in clinically recognized pregnancies is less than 5 percent in women under 30, but as high as 35 percent in women over the age of 42 (Figure 19-38)! The reason for this dramatic increase in nondisjunction with maternal age lies in the biology of female meiosis in vertebrates. In all vertebrates, pre-