Classic Experiment 19-2: Synthesis and Degradation of Mitotic Cyclin Are Required for Progression through Mitosis
Synthesis and Degradation of Mitotic Cyclin Are Required for Progression through Mitosis
W. Murray and M. W. Kirschner, 1989, Nature 339:275
W. Murray, M. J. Solomon, and M. W. Kirschner, 1989, Nature 339:280
Background
Tim Hunt and Joan Ruderman identified a class of proteins, which they called cyclins, in surf clams and sea urchin embryos. These proteins were peculiar in that their abundance fluctuated during the cell cycle (see Classic Experiment 19-1). Cyclins were synthesized during interphase, peaked during mitosis, and were abruptly degraded shortly thereafter. The behavior of these cyclin proteins was interesting in light of previous studies by Yoshio Matsui. He had identified an activity in Xenopus eggs that had the ability to induce resting G2 Xenopus oocytes to enter meiosis. Matsui called this activity maturation promoting factor (MPF). Could it be that the cyclin proteins whose abundance peaked in mitosis were constituents of this activity? Andrew Murray and Marc Kirschner set out to test this idea (Murray and Kirschner, 1989). For their experiments, they used Xenopus oocyte extracts. As described in Section 19.2, large amounts of Xenopus oocytes can be harvested and extract prepared with ease, making this model system ideally suited for biochemical investigation of the cell cycle.
The Experiment
Cytoplasmic extracts prepared from unfertilized Xenopus eggs contain all the materials (mRNAs and proteins) required for multiple cell cycles. When nuclei prepared from Xenopus sperm (sperm nuclei were used in this experiment because they are readily isolated in large numbers) are added to such an egg extract, the nuclei in the egg extract are induced to behave as if progressing through the cell cycle. That is, they replicate their DNA and then undergo mitosis in the extract. Under normal conditions, cyclins peak during early mitosis, then are rapidly degraded (Figure 1a).
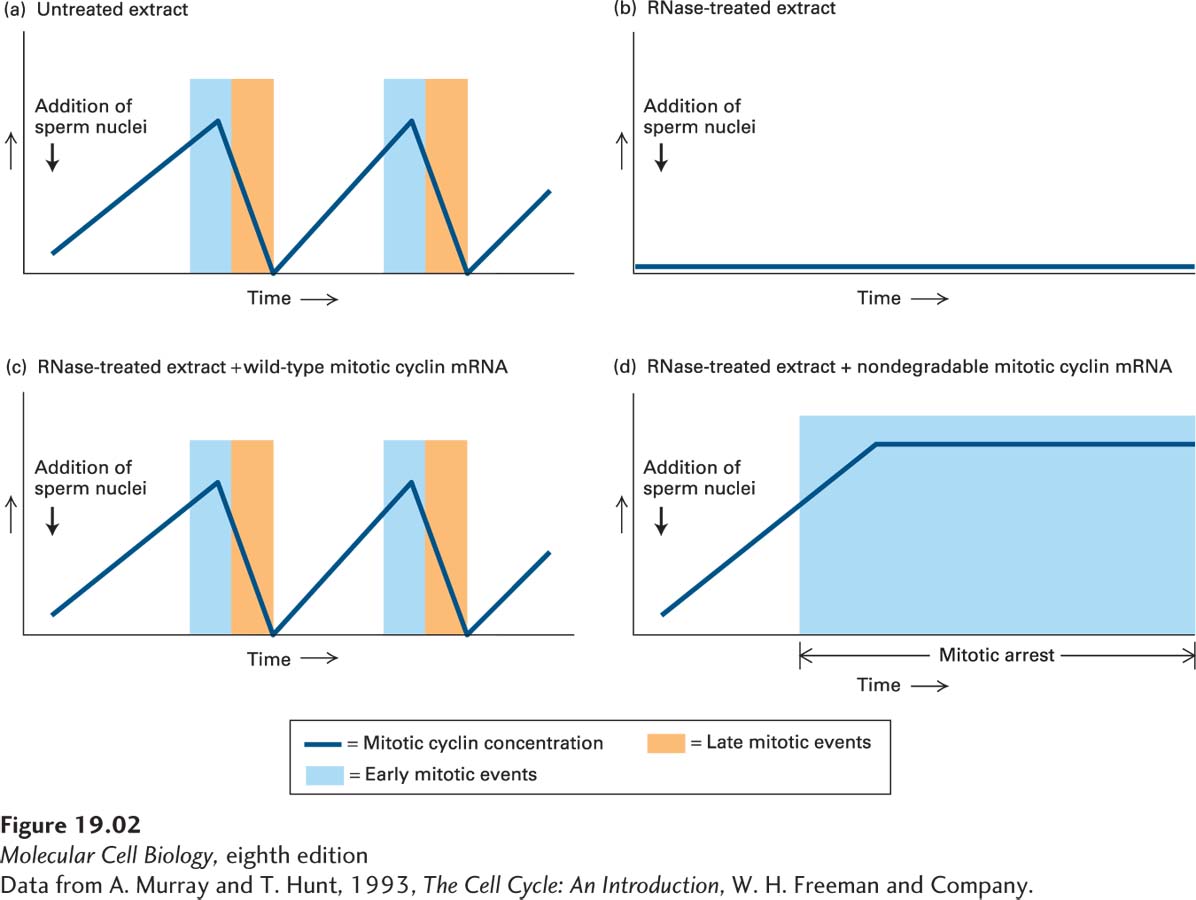
Murray and Kirschner wanted to determine whether the cyclins that Hunt and Ruderman had identified, like MPF, had the ability to induce mitosis. Because cyclins appeared to be unstable at the end of mitosis, Murray and Kirschner reasoned that removing the mRNA from the egg extract would prevent the new synthesis of all unstable proteins, including the cyclins, in the next cell cycle (all other stable proteins would still be present in the extract). To do this, they digested all mRNAs with a low concentration of RNase, which was then inactivated by the addition of a specific inhibitor. This treatment destroys mRNAs without affecting the tRNAs and rRNAs that are required for protein synthesis. When sperm nuclei were added to the RNase-
In a second set of experiments, Murray, Solomon and Kirschner found that cyclins not only limit entry into mitosis, but must be degraded to allow exit from mitosis (Murray et al., 1989). Recall that in Hunt’s and Ruderman’s experiments, not only did the accumulation of cyclins coincide with entry into mitosis, but their disappearance occurred concomitantly with exit from mitosis. This finding raised the possibility that mitotic cyclin degradation was necessary for cells to exit from mitosis. To test this possibility, Murray, Solomon and Kirschner again used Xenopus egg extracts, but instead of adding wild-
Discussion
The cyclin that Hunt and Ruderman had identified was the founding member of a family of proteins that we now know regulate the activity of cyclin-
The initial study by Murray and Kirschner also illustrated the importance of restricting cyclins to the appropriate cell cycle stage. The mechanisms a cell employs to accomplish this restriction are described in Section 19.4. The study of how cyclins are eliminated at the end of mitosis further revealed the critical importance of ubiquitin-