Classic Experiment 19-3: The Formulation of the Checkpoint Concept
The Formulation of the Checkpoint Concept
T. A. Weinert and L. H. Hartwell, 1988, Science 241:317
Background
Hartwell and colleagues identified temperature-
The Experiment
The Cdc13 protein is required for telomere replication, and in its absence, large stretches of incompletely replicated telomeric DNA persist in cells. Cells carrying a temperature-
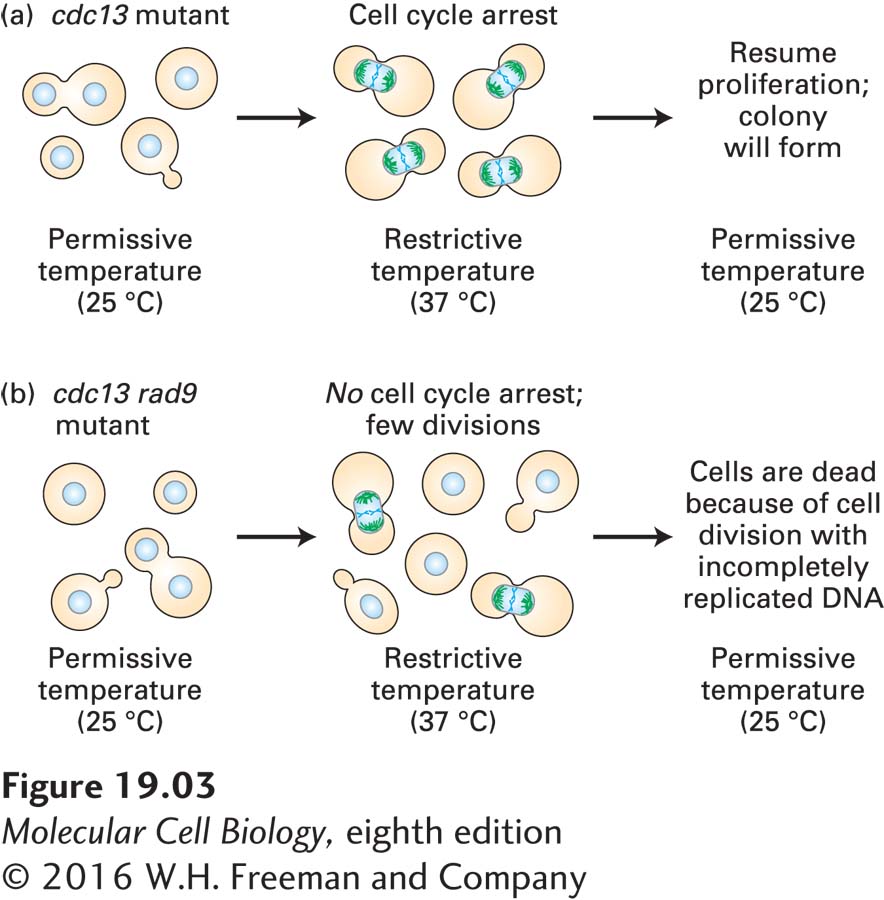
To characterize the cdc13 mutant in more detail, Weinert and Hartwell examined the effects of introducing a second mutation in another gene, a deletion in the RAD9 gene (Weinert and Hartwell, 1988). The RAD9 gene is not essential for viability, but when it is deleted, cells are extremely sensitive to DNA-
Weinert and Hartwell proposed the following explanation for this observation: the cdc13 mutants arrest at the restrictive temperature because they harbor incompletely replicated DNA. This damaged DNA signals the cell to arrest cell cycle progression and induce repair of the damage because mitosis of cells with damaged DNA would almost certainly lead to cell death. The RAD9 gene is part of the machinery that conveys this “halt cell cycle progression” signal. In cells lacking Rad9, this signal does not work, and cells undergo mitosis despite incompletely replicated DNA, which kills the cells. Weinert and Hartwell called this surveillance mechanism a checkpoint pathway.
Discussion
The checkpoint pathway concept had a profound effect not only on cell cycle research, but on all biological research. We now know that cell cycle progression is monitored by multiple surveillance mechanisms that ensure that cells grow to the appropriate size before they begin to divide, that their chromosomes are attached correctly to the spindle before they commence chromosome condensation, and that the nucleus is correctly positioned in the cell before they undergo cytokinesis. Checkpoint pathways also play critical roles during development. They ensure that subsequent developmental steps are not initiated before a prior step has been completed.
Importantly, checkpoint pathways, especially the DNA damage checkpoint pathway, are often inactivated in cancer, illustrating their importance for cellular and organismal function. The checkpoint concept is a powerful example of how studies in model organisms provide fundamental insights into all of biology as well as important insights into human diseases.