Embryonic Development Uses a Conserved Set of Master Transcription Factors
The astute reader will note a paradox in the previous discussion: if indeed most human protein-coding genes are shared with apes and mice, and many with flies and worms, how is it that these organisms look and function so differently? The answer to this question resides in the way genes are regulated during the development of all metazoans from a single cell, the fertilized egg. As we learn in Chapters 8 and 9, each protein-coding gene is associated with regulatory DNA sequences that differ in different organisms. Many of these regulatory sequences bind proteins that direct the expression of the gene, and thus the amount of a protein it makes, in specific types of cells. Some of these proteins are termed master transcription factors; these proteins bind to regulatory DNA sequences, are conserved throughout evolution, and control the development of specific types of cells by activating or repressing groups of genes, often at different stages of development.
The early stages in the development of a human embryo are similar to those in the mouse. They are characterized by rapid cell divisions (Figure 1-28) followed by the differentiation of cells into tissues. In all organisms, the embryonic body plan—the spatial pattern of cell types (tissues) and body parts—emerges from two influences: a program of genes that specifies the pattern of the body, and local cell interactions that induce different parts of the program.
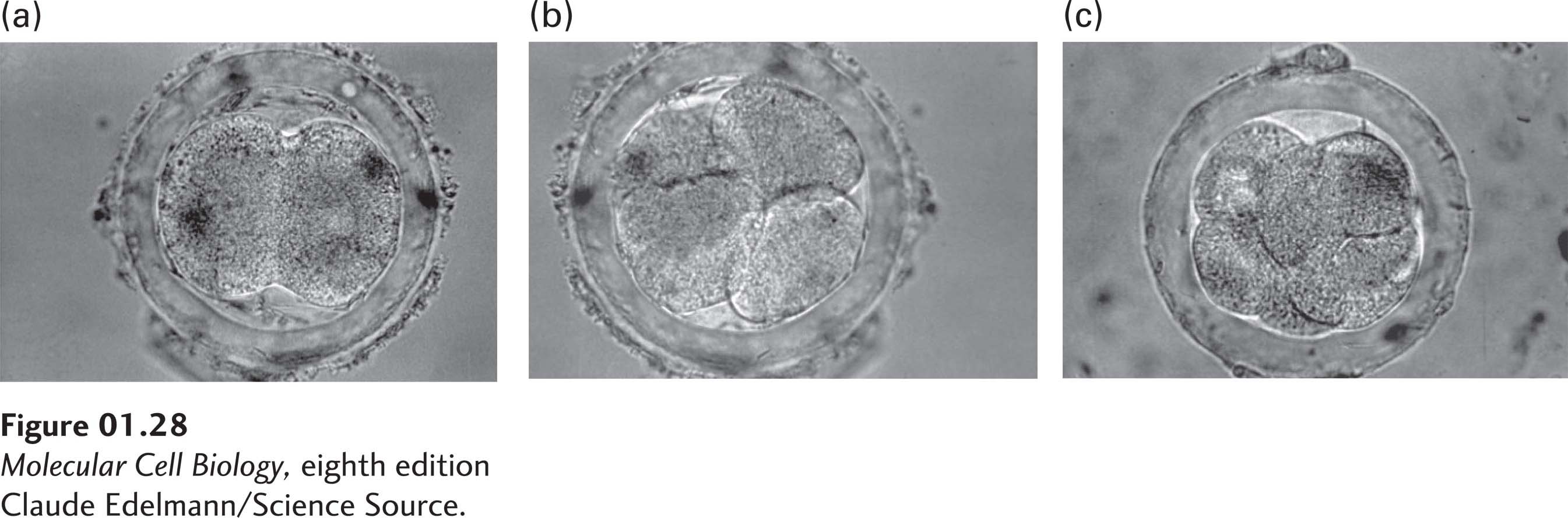
FIGURE 1-28 The first few cell divisions of a fertilized egg set the stage for all subsequent development. A developing mouse embryo is shown at the (a) two-cell, (b) four-cell, and (c) eight-cell stages. The embryo is surrounded by supporting membranes. The corresponding steps in human development occur during the first few days after fertilization.
[Claude Edelmann/Science Source.]
With only a few exceptions, animals display axial symmetry; that is, their left and right sides mirror each other. This most basic of patterns is encoded in the genome. Developmental biologists have divided bilaterally symmetric animal phyla into two large groups depending on where the mouth and anus form in the early embryo. Protostomes develop a mouth close to a transient opening in the early embryo (the blastopore) and have a ventral nerve cord; protostomes include all worms, insects, and mollusks. Deuterostomes develop an anus close to this transient opening in the embryo and have a dorsal central nervous system; they include echinoderms (such as sea stars and sea urchins) and vertebrates. The bodies of both protostomes and deuterostomes are divided into discrete segments that form early in embryonic development. Protostomes and deuterostomes probably evolved from a common ancestor, termed Urbilateria, that lived approximately 600 million years ago (Figure 1-29a).
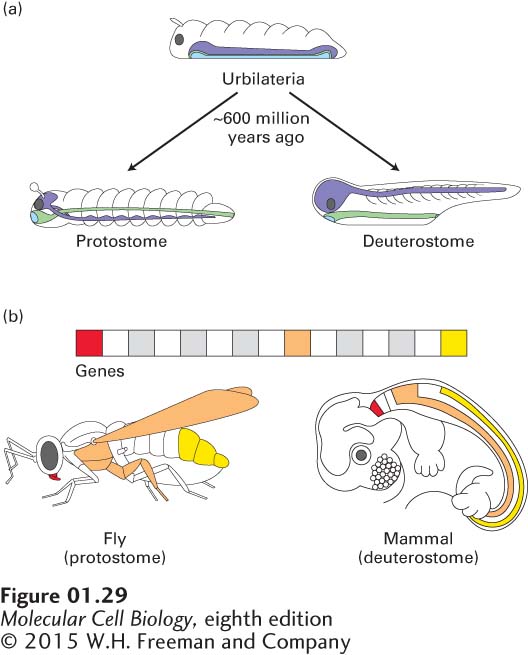
FIGURE 1-29 Similar master transcription factors, conserved during evolution, regulate early developmental processes in diverse animals. (a) Urbilateria is the presumed ancestor of all protostomes and deuterostomes that existed about 600 million years ago. The positions of its nerve cord (violet), surface ectoderm (mainly skin; white), and endoderm (mainly digestive tract and organs; light green) are shown. (b) Highly conserved master transcription factors called Hox proteins, which determine the identity of body segments during embryonic development, are found in both protostomes and deuterostomes. Hox genes are found in clusters on the chromosomes of most or all animals, and they encode related master transcription factors that control the activities of other genes. In many animals, different Hox genes direct the development of different segments along the head-to-tail axis, as indicated by corresponding colors. Each gene is activated (transcriptionally) in a specific region along the head-to-tail axis and controls the growth and development of tissues there. For example, in the mouse, a deuterostome, the Hox genes are responsible for the distinctive shapes of vertebrae. Mutations affecting Hox genes in the fruit fly, a protostome, cause body parts to form in the wrong locations, such as legs in lieu of antennae on the head. In both organisms, these genes provide a head-to-tail “address” and serve to direct the formation of structures in the appropriate places.
Many patterning genes encode master transcription factors that control expression of other genes and specify the general organization of an organism, beginning with the major body axes—anterior-posterior (head-to-tail), dorsal-ventral (back-to-belly), and left-right—and ending with body segments such as the head, chest, abdomen, and tail. The conservation of axial symmetry from the simplest worms to mammals is explained by the presence of conserved patterning genes in their genomes. Other patterning genes encode proteins that are important in cell adhesion or in cell signaling. This broad repertoire of patterning genes permits the integration and coordination of events in different parts of the developing embryo and gives each segment in the body its unique identity.
Remarkably, many patterning genes encoding master transcription factors are highly conserved in both protostomes and deuterostomes (Figure 1-29b). This conservation of body plan reflects evolutionary pressure to preserve the commonalities in the molecular and cellular mechanisms controlling development in different organisms. For instance, fly eyes and human eyes are very different in their structure, function, and nerve connections. Nonetheless, the master transcription factors that initiate eye development—eyeless in the fly and Pax6 in the human—are highly related proteins that regulate the activities of other genes and are descended from the same ancestral gene. Mutations in the eyeless or Pax6 genes cause major defects in eye formation (Figure 1-30).
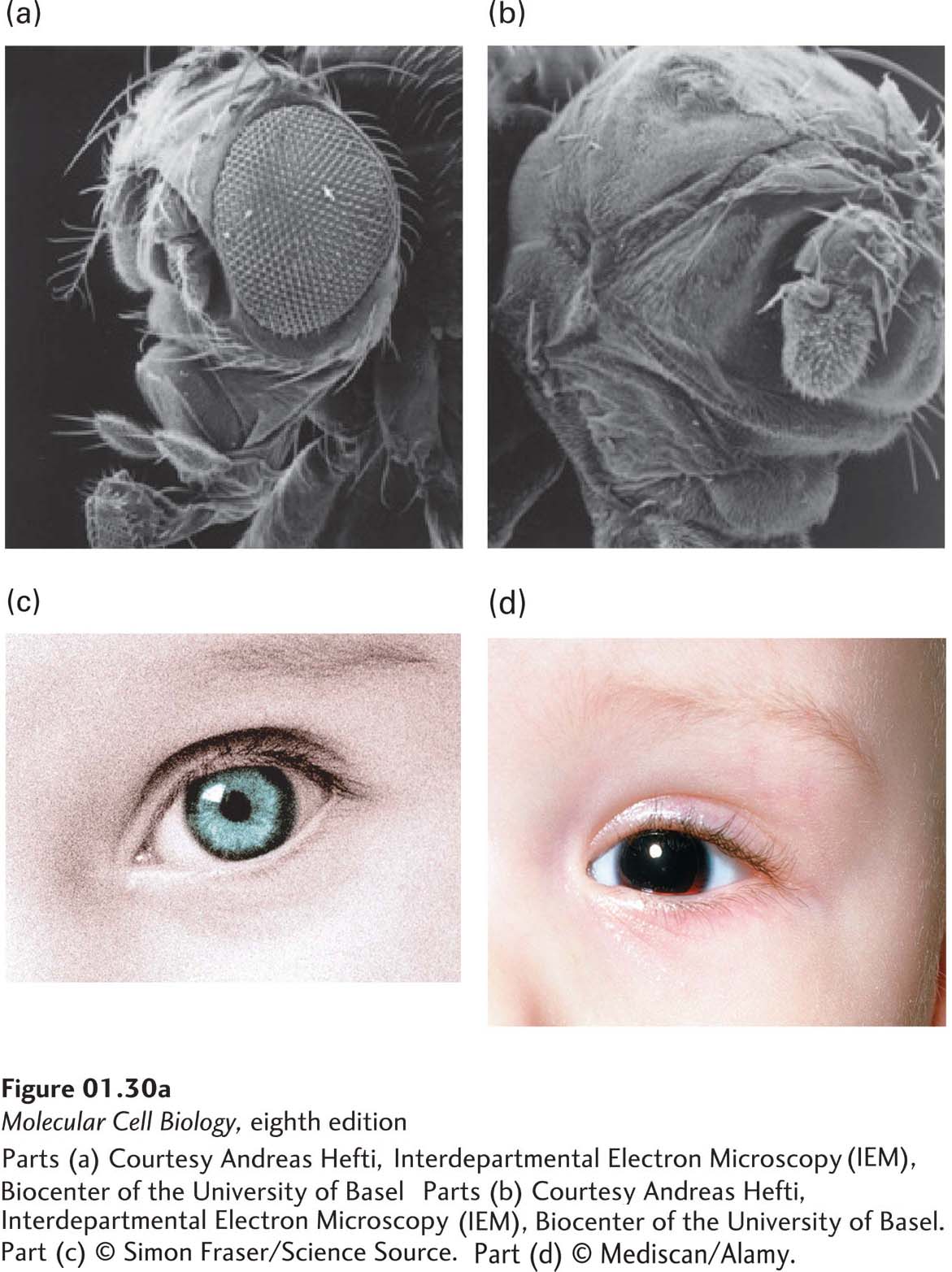
FIGURE 1-30 Homologous genes regulate eye development in diverse animals. (a) Development of the large compound eyes in fruit flies requires a gene called eyeless (named for the mutant phenotype). (b) Flies with inactivated eyeless genes lack eyes. (c) Normal human eyes require the gene Pax6, the homolog of eyeless. (d) People lacking adequate Pax6 function have the genetic disease aniridia, a lack of irises in the eyes. Pax6 and eyeless, which encode highly related master transcription factors that regulate the activities of other genes, are homologs and presumably descended from the same ancestral gene.
[Parts (a) and (b) Courtesy Andreas Hefti, Interdepartmental Electron Microscopy (IEM), Biocenter of the University of Basel. Part (c) © Simon Fraser/Science Source. Part (d) © Mediscan/Alamy.]


