Tight Junctions Seal Off Body Cavities and Restrict Diffusion of Membrane Components
For polarized epithelial cells to function as barriers and mediators of selective transport, extracellular fluids surrounding their apical and basolateral membranes must be kept separate. Tight junctions between adjacent epithelial cells are usually located in a band surrounding the cell just below the apical surface (Figure 20-17; see also Figure 20-11). These specialized junctions form a barrier that seals off body cavities such as the intestinal lumen and separates the blood from the cerebral spinal fluid of the central nervous system (i.e., the blood-brain barrier).
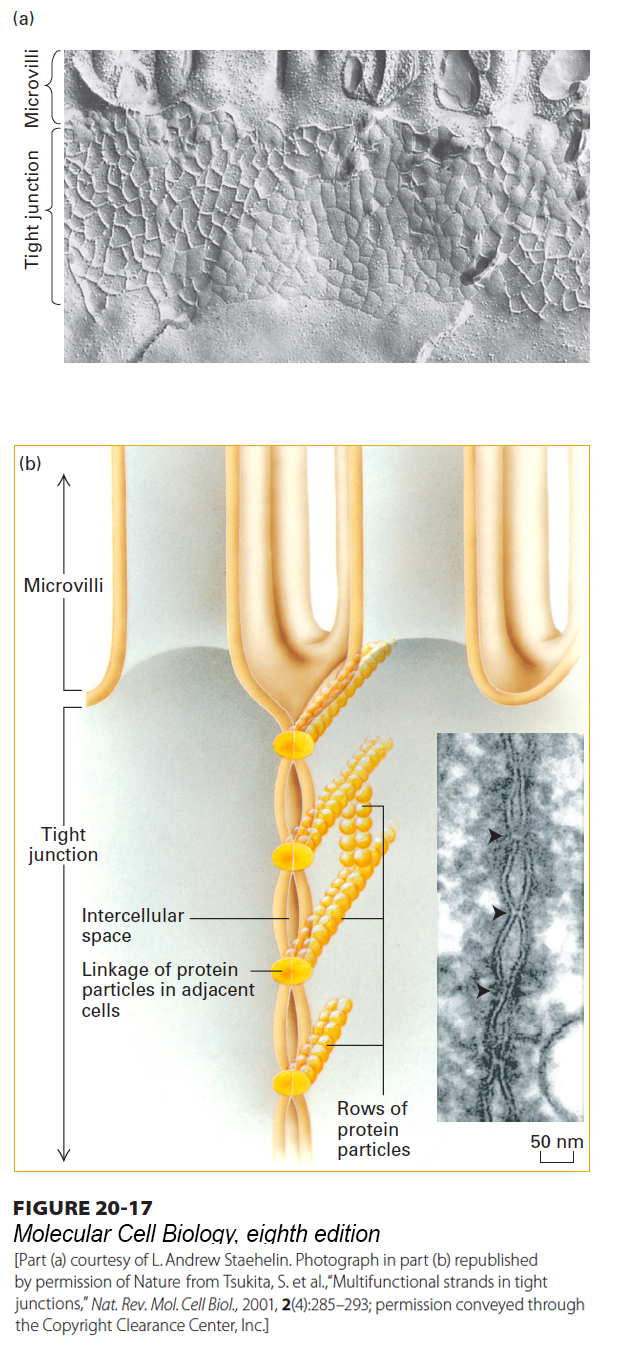
FIGURE 20-17 Tight junctions. (a) Freeze-fracture preparation of tight junction zone between two intestinal epithelial cells. The fracture plane passes through the plasma membrane of one of the two adjacent cells (see also Figure 20-11). A honeycomb-like network of ridges and grooves below the microvilli constitutes the tight-junction zone. (b) Schematic drawing shows how a tight junction might be formed by the linkage of rows of protein particles in adjacent cells. In the inset micrograph of an ultrathin sectional view of a tight junction, the adjacent cells can be seen in close contact where the rows of proteins interact. See L. A. Staehelin and B. E. Hull, 1978, Sci. Am. 238:140, and D. Goodenough, 1999, P. Natl. Acad. Sci. USA 96:319.
[Part (a) courtesy of L. Andrew Staehelin. Photograph in part (b) republished by permission of Nature, from Tsukita, S. et al., “Multifunctional strands in tight junctions,” Nat. Rev. Mol. Cell Biol., 2001, 2(4):285–293; permission conveyed through the Copyright Clearance Center, Inc.]
Tight junctions prevent the diffusion of macromolecules and, to varying degrees, small water-soluble molecules and ions across an epithelium via the spaces between cells. They also help establish and maintain the polarity of epithelial cells by preventing the diffusion of membrane proteins and glycolipids between the apical and the basolateral regions of the plasma membrane, ensuring that these regions contain different membrane components. Indeed, the lipid compositions of the apical and basolateral regions of the plasma membrane’s exoplasmic leaflet (see Chapter 7) are distinct. Essentially all cell surface glycolipids are restricted to the exoplasmic face of the apical membrane, as are all proteins linked to the membrane by a glycosylphosphatidylinositol (GPI) anchor (see Figure 7-19). In contrast, the apical and basolateral regions of the plasma membrane’s cytosolic leaflet have uniform membrane composition in epithelial cells; their lipids and proteins can apparently diffuse laterally from one region of the membrane to the other.
Tight junctions are composed of thin bands of plasma-membrane proteins that completely encircle the cell and are in contact with similar thin bands on adjacent cells. When thin sections of the tight junctions in cells are viewed in an electron microscope, the lateral surfaces of adjacent cells appear to touch each other at intervals and even to fuse in the zone just below the apical surface (see Figure 20-11b). In freeze-fracture preparations, tight junctions appear as an interlocking network of ridges and grooves in the plasma membrane (Figure 20-17a). Very high magnification reveals that rows of protein particles 3–4 nm in diameter form the ridges seen in freeze-fracture micrographs of tight junctions. In the model shown in Figure 20-17b, the tight junction is formed by a double row of these particles, one row donated by each cell. Treatment of an epithelium with the protease trypsin destroys the tight junctions, supporting the proposal that proteins are essential structural components of these junctions.
The two principal integral membrane proteins found in tight junctions are occludin and claudin (from the Latin claudere, “to close”). When investigators engineered mice with mutations inactivating the occludin gene, which was thought to be essential for tight-junction formation, the mice surprisingly still had morphologically distinct tight junctions. Further analysis led to the discovery of claudin. Each of these proteins has four membrane-spanning α helices (Figure 20-18). The mammalian claudin gene family encodes at least 27 homologous proteins that exhibit distinct tissue-specific patterns of expression. A group of junction adhesion molecules (JAMs) have also been found to contribute to homophilic adhesion and other functions of tight junctions. JAMs and another junctional protein, the coxsackievirus and adenovirus receptor (CAR), contain a single transmembrane α helix and belong to the Ig superfamily of CAMs. The extracellular domains of rows of occludin, claudin, and JAMs in the plasma membrane of one cell apparently form extremely tight links with similar rows of the same proteins in an adjacent cell, creating a tight seal. Ca2+-dependent cadherin-mediated adhesion also plays an important role in tight-junction formation, stability, and function.
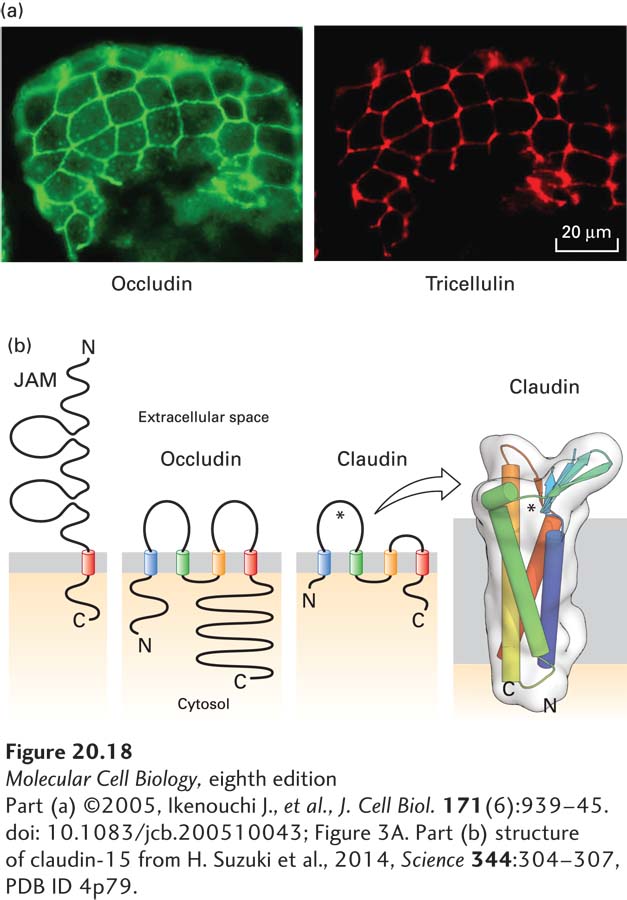
FIGURE 20-18 Proteins that compose tight junctions. (a) Immunofluorescence localization of occludin (green) and tricellulin (red) in mouse intestinal epithelium. Note that tricellulin is predominantly concentrated in tricellular junctions. (b) The junction adhesion molecule (JAM) has a single transmembrane domain and an extracellular region with two immunoglobulin domains, whereas occludin and claudins contain four transmembrane helices. The larger extracellular loop of the claudins, indicated by an asterisk, contributes to paracellular ion selectivity. The transmembrane helices of claudin-15, which permits paracellular transport of cations, form a four-helix bundle, and the extracellular loops contain a five-stranded β sheet (seen edgewise in this view). This β sheet has been proposed to help define the pore through which ions pass (near the asterisk). See S. Tsukita et al., 2001, Nat. Rev. Mol. Cell Biol. 2:285.
[Part (a) ©2005, Ikenouchi J., et al., J. Cell Biol. 171(6):939–45. doi: 10.1083/jcb.200510043; Figure 3A. Part (b) structure of claudin-15 from H. Suzuki et al., 2014, Science 344:304–307, PDB ID 4p79.]
At the intersection of three cells connected to one another by tight junctions (see Figure 20-13 and Figure 20-18a), two additional transmembrane proteins are incorporated into the tight junctions: tricellulin, which has four membrane-spanning helices, as do occludin and claudins, and angulins, which have a single transmembrane helix and one extracellular immunoglobulin domain and which appear to be required for the assembly of tricellulin where the cells intersect.
As is the case for adherens junctions and desmosomes, cytosolic adapter proteins and their connections to the cytoskeleton are critical components of tight junctions. For example, the long C-terminal cytosolic segment of occludin binds to PDZ domains in some large, multidomain adapter proteins. PDZ domains are about 80 to 90 amino acids long and are found in various cytosolic proteins; they mediate binding to other cytosolic proteins or to the C-termini of particular plasma-membrane proteins. Cytosolic proteins containing a PDZ domain often have more than one of them. In the human genome, there are more than 250 PDZ domains in hundreds of proteins. Proteins with multiple PDZ domains can serve as scaffolds on which to assemble proteins into larger functional complexes. Several multiple-PDZ-domain–containing adapter proteins are associated with tight junctions, including the zonula occludens (ZO) proteins ZO-1, ZO-2, and ZO-3, which not only interact with occludin, claudin, and other adapter and signaling proteins but also mediate association with actin fibers. These interactions appear to stabilize the linkage between occludin and claudin molecules that is essential for maintaining the integrity of tight junctions. ZO proteins can also function as adapters for adherens junctions (see Figure 20-14) and gap junctions.
A simple experiment demonstrates the impermeability of tight junctions to some water-soluble substances. In this experiment, lanthanum hydroxide (an electron-dense colloid of high molecular weight) is injected into the pancreatic blood vessel of an experimental animal; a few minutes later, the pancreatic epithelial acinar cells are fixed and prepared for microscopy. As shown in Figure 20-19, the lanthanum hydroxide diffuses from the blood into the space that separates the lateral surfaces of adjacent acinar cells, but cannot penetrate past the tight junction.
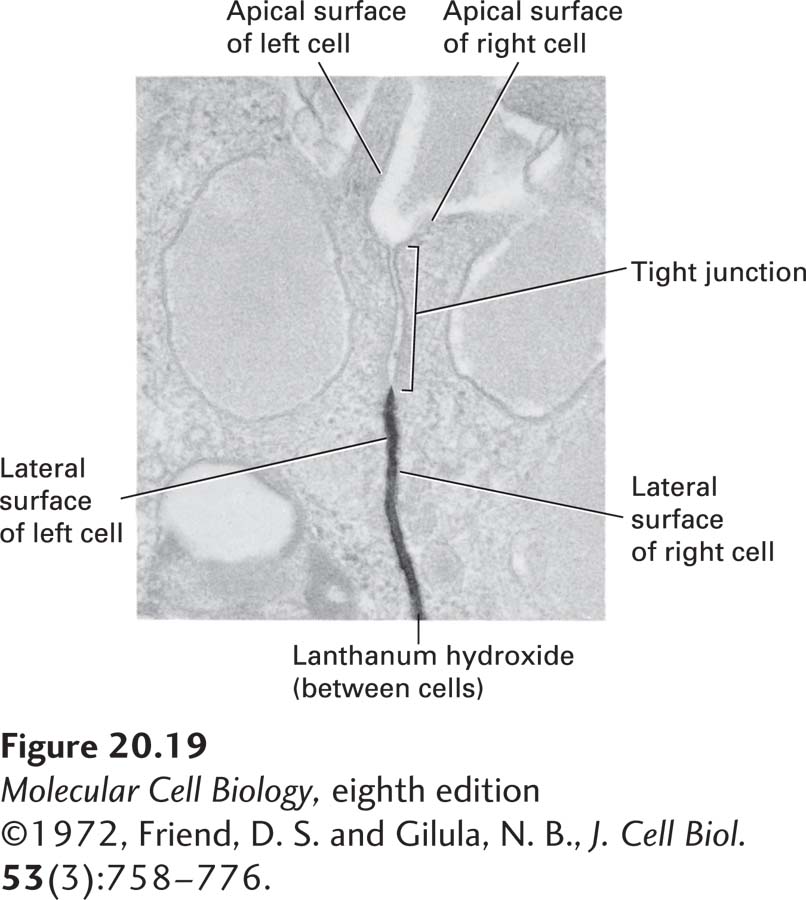
EXPERIMENTAL FIGURE 20-19 Tight junctions prevent passage of large molecules through extracellular spaces between epithelial cells. Tight junctions in the pancreas are impermeable to the large water-soluble colloid lanthanum hydroxide (dark stain) administered from the basolateral side of the epithelium.
[©1972, Friend, D. S. and Gilula, N. B., J. Cell Biol. 53(3):758–776.]
As a consequence of tight junctions, many nutrients cannot move across the intestinal epithelium between cells; instead, their transport is achieved in large part through the transcellular pathway via specific membrane-bound transport proteins (Figure 20-20; see also Figure 11-30). The barrier to diffusion provided by tight junctions, however, is not absolute, for they exhibit size- and ion-selective permeability. Thus certain small molecules and ions can move from one side of the epithelium to the other through the paracellular pathway (see Figure 20-20). The importance of selective permeability is highlighted by the evolutionary conservation of the molecules that establish it and the diseases that arise when it is disrupted. For example, murine embryos cannot develop properly if selective permeability is disrupted because proper fluid balance on the two sides of epithelia cannot be maintained. Similarly, the kidneys depend on proper tight-junction permeability to establish the ion gradients necessary for normal regulation of body fluids and waste removal. Owing at least in part to the varying properties of the different types of claudin molecules located in different tight junctions, the permeability of the tight junctions to ions, small molecules, and water varies enormously among different epithelial tissues. The large extracellular loop in the claudins (see Figure 20-18) is thought to play a major role in defining the selective permeability conferred on tight junctions by specific claudin isoforms.
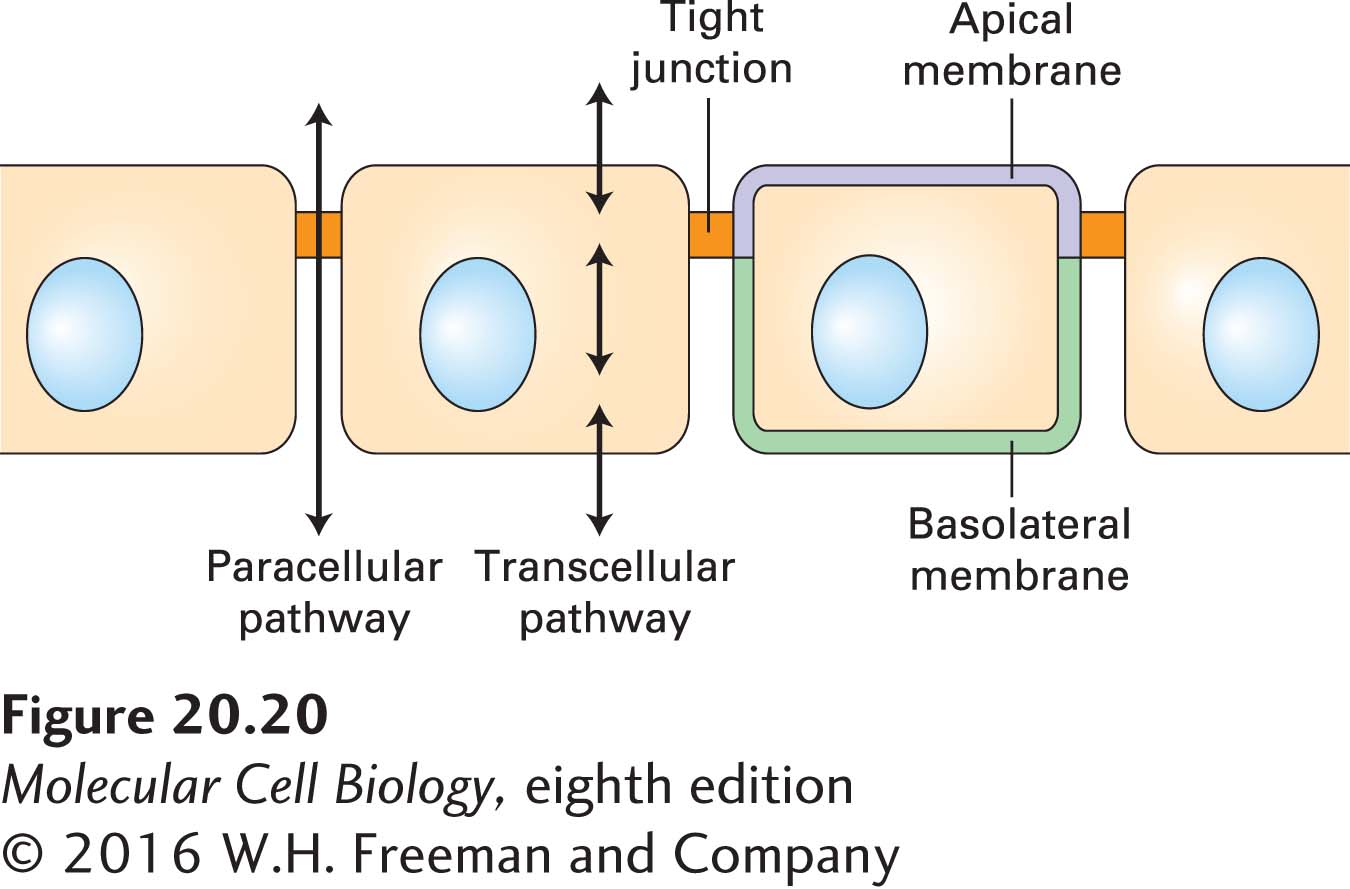
FIGURE 20-20 Transcellular and paracellular pathways of transepithelial transport. Transcellular transport requires the cellular uptake of molecules on one side and subsequent release on the opposite side by mechanisms discussed in Chapter 11. In paracellular transport, molecules move extracellularly through parts of tight junctions, whose permeability to small molecules and ions depends on the composition of the junctional components and the physiological state of the epithelial cells. See S. Tsukita et al., 2001, Nat. Rev. Mol. Cell Biol. 2:285.
The permeability of tight junctions can be altered by intracellular signaling pathways, especially G protein and cyclic AMP–coupled pathways (see Chapter 15). The regulation of tight-junction permeability is often studied by measuring ion flux (electrical resistance, called transepithelial resistance) or the movement of radioactive or fluorescent molecules across monolayers of MDCK or other epithelial cells.
The importance of paracellular transport is apparent in several human diseases. In hereditary hypomagnesemia, a defect in the claudin16 gene prevents the normal paracellular flow of magnesium in the kidney. This defect results in an abnormally low blood level of magnesium, which can lead to convulsions. Furthermore, a mutation in the claudin14 gene causes hereditary deafness, apparently by altering transport around hair-cell epithelia in the cochlea of the inner ear.
Some pathogens have evolved to exploit the molecules in tight junctions. Some use junctional proteins as “co-receptors” to attach to cells prior to infecting them (e.g., hepatitis C virus uses claudin-1 and occludin, together with two other co-receptors, to enter liver cells). Others break down the tight-junction barrier and cross epithelia via paracellular movement, and still others produce toxins that alter barrier function. For example, toxins produced by Vibrio cholerae, the enteric bacterium that causes cholera, alter the permeability barrier of the intestinal epithelium by altering the composition or activity of tight junctions. Vibrio cholerae also releases a protease that disrupts tight junctions by degrading the extracellular domain of occludin. Other bacterial toxins can affect the ion-pumping activity of membrane transport proteins in intestinal epithelial cells. Toxin-induced changes in tight-junction permeability (increased paracellular transport) and in protein-mediated ion pumping (increased transcellular transport) can result in massive losses of internal body ions and water into the gastrointestinal tract, which in turn leads to diarrhea and potentially lethal dehydration (see Chapter 11).



