Laminin, the principal multi-adhesive matrix protein in basal laminae, is a heterotrimeric protein comprising α, β, and γ chains. At least 16 laminin isoforms in vertebrates are assembled from 5 α, 3 β, and 3 γ chains, with each chain numbered to reflect the chain composition: laminin-111 (α1β1γ1) or laminin-511 (α5β1γ1). Each laminin isoform exhibits a distinctive pattern of tissue- and developmental stage–specific expression. As shown in Figure 20-24, many laminins are large, cross-shaped proteins (molecular weight of about 820,000), although some are Y or rod shaped. Globular domains at the N-terminus of each subunit bind to one another and thus mediate the self-assembly of laminins into mesh-like networks. Five globular LG domains at the C-terminus of the laminin α subunit mediate Ca2+-dependent binding to cell-surface laminin receptors, including certain integrins (see Table 20-4) as well as sulfated glycolipids, syndecan, and dystroglycan, which will be described further in Section 20.4. Some of these interactions are via negatively charged carbohydrates on the receptors. LG domains are found in a wide variety of other proteins and can mediate binding to steroids and proteins as well as carbohydrates. Laminin is the principal basal laminal ligand of integrins.
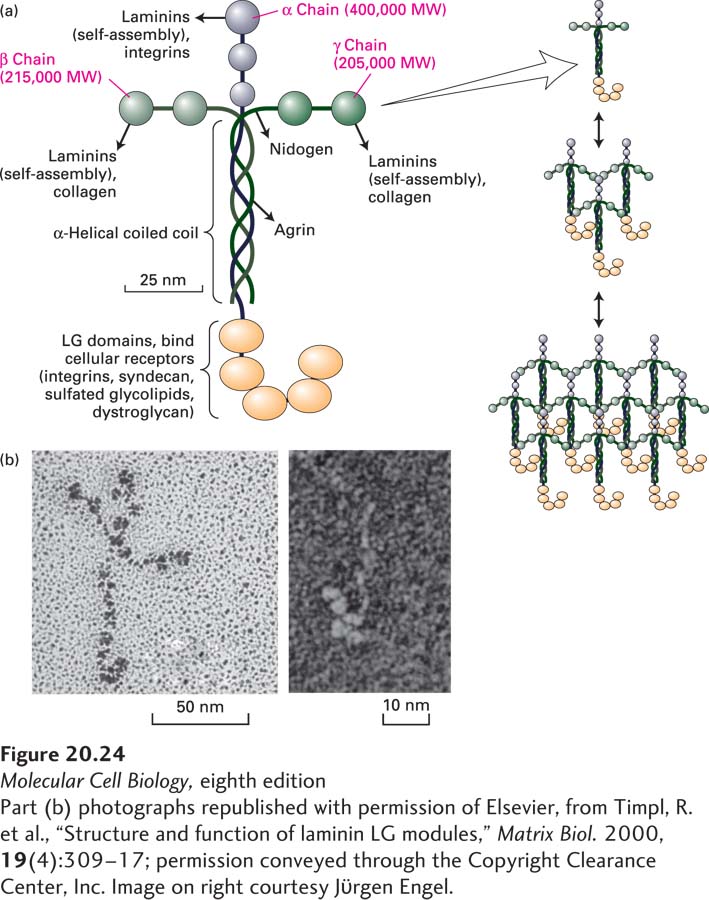
FIGURE 20-24 Laminin is a heterotrimeric multi-adhesive matrix protein found in all basal laminae. (a) Schematic model of cross-shaped laminin molecule showing the general shape, location of globular domains, and coiled-coil region in which laminin’s three chains are covalently linked by several disulfide bonds. Different regions of laminin bind to adhesion receptors and various matrix components (indicated by arrows). Right: Laminins assemble into a lattice via interactions between their N-terminal globular domains. See G. R. Martin and R. Timpl, 1987, Annu. Rev. Cell Biol. 3:57; M. Durbeej, 2010, Cell Tissue Res. 339:259–268; and S. Meinen et al., 2007, J. Cell Biol. 176:979–993. (b) Electron micrographs of an intact laminin molecule, showing its characteristic cross shape (left), and the carbohydrate-binding LG domains near the C-terminus (right).
[Part (b) photographs republished with permission of Elsevier, from Timpl, R. et al., “Structure and function of laminin LG modules,” Matrix Biol. 2000, 19(4):309–17; permission conveyed through the Copyright Clearance Center, Inc. Image on right courtesy Jϋrgen Engel.]

