Mechanotransduction is the reciprocal interconversion of a mechanical force—or stimulus—and biochemical processes. These interconversions underlie a variety of biological activities, such as signaling, regulated gene expression, cell proliferation, cell migration, and interactions among cells and between cells and the ECM. Mechanotransduction in the context of cell-cell and cell-ECM interactions usually involves a cell-surface CAM or adhesion receptor that transmits mechanical force or biochemical information across the plasma membrane and one or more intracellular or extracellular mechanosensors that respond to the mechanical stimulus by changing shape and activity (see also Chapter 22). For example, tension applied across the length of a multidomain mechanosensor protein, such as the ECM protein fibronectin or the integrin adapter protein talin, can literally pull apart one or more domains, thereby exposing binding sites that were otherwise inaccessible (cryptic) in the folded domain (Figure 20-9). The newly accessible binding sites can then recruit binding partners—in some cases after phosphorylation—and alter cellular or extracellular functions. For example, the stretching of fibronectins by integrins induces their assembly into fibrils, which in some cases is an early step in the assembly of collagen and other molecules into ECM. The mechanical forces in mechanotransduction can be forces generated within a cell, such as myosin-driven movement of actin filaments (Chapter 17), or outside a cell, such as blood flow, movement of adjacent cells, or contraction or expansion of ECM.
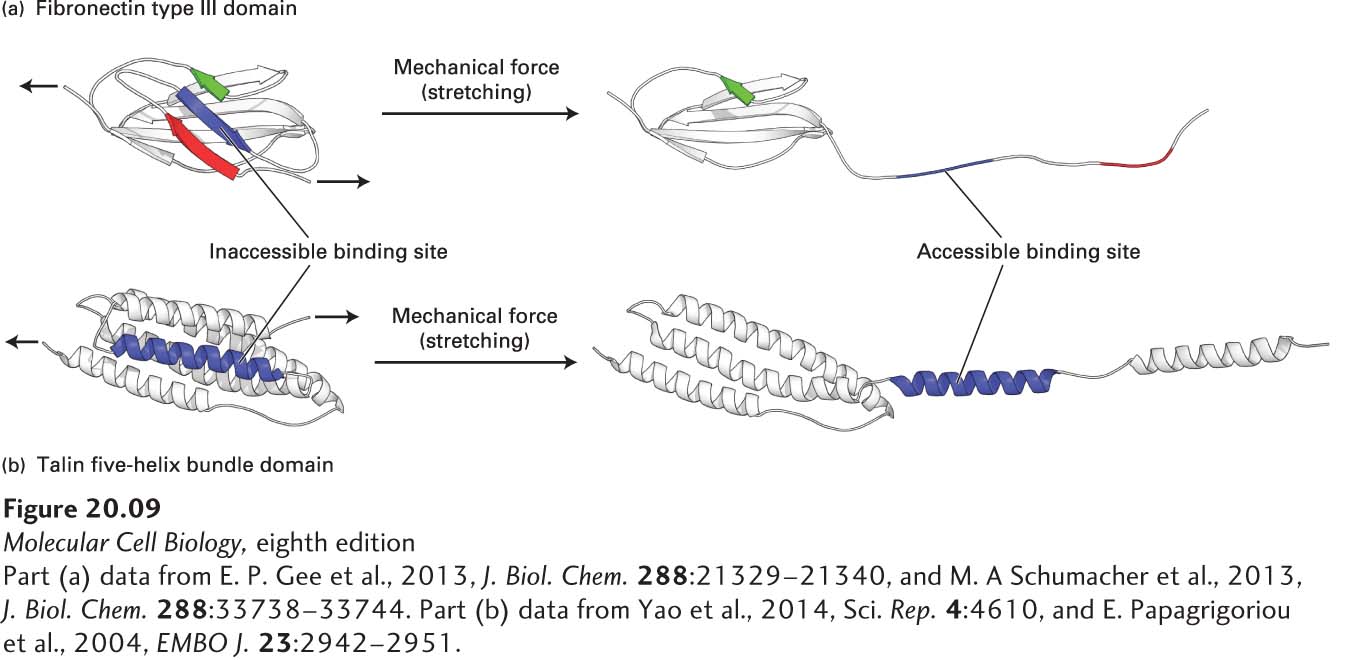
FIGURE 20-9 Models of Domains in Mechanosensor Proteins Responding to Mechanical Forces. (a) Hypothetical model of the partial unfolding of a fibronectin type III domain in the ECM molecule fibronectin when that protein is subjected to mechanical force. Mechanical force generated within the cell by actin movement and mechanotransduced via multiple integrin adhesion receptors bound to the extracellular dimeric fibronectin can partially unfold the fibronectin. The unfolding is thought to expose a putative, previously hidden (cryptic) binding site on fibronectin (blue segment) that has the potential to form β sheets with other fibronectin molecules, recruiting them to form fibronectin fibrils, and thus helping assemble the ECM. (b) Hypothetical model of the partial unfolding of a domain (the R1 five-helix bundle) in the intracellular integrin adapter protein talin when it is subjected to mechanical stretching force. This force is generated by actin, which can bind to and pull on the C-terminus of talin while talin’s N-terminus is bound to the cytoplasmic tail of integrin’s β subunit. The unfolding is thought to expose this domain’s otherwise cryptic α-helical vinculin binding site (blue). Vinculin, an actin-binding protein (see Figure 20-14d), can then bind to the integrin-talin complex via the exposed site and in turn bind to actin, thus promoting the assembly of multiple actin fibers. The assembly of actin fibers indirectly linked by adapters to integrins strengthens integrin-mediated adhesion and helps to build focal adhesions.
[Part (a) data from E. P. Gee et al., 2013, J. Biol. Chem. 288:21329–21340, and M. A Schumacher et al., 2013, J. Biol. Chem. 288:33738–33744. Part (b) data from Yao et al., 2014, Sci. Rep. 4:4610, and E. Papagrigoriou et al., 2004, EMBO J. 23:2942–2951.]

