ES and iPS Cells Can Generate Functional Differentiated Human Cells
Neurons and glial cells, as well as other cell types, derived from human iPS cells have been implanted into mice with some promising results. Stem cell–derived cardiomyocytes (heart muscle cells) can correct heart arrhythmias; certain glial cells—oligodendrocytes—show promise in aiding recovery from experimental spinal injury; and retinal epithelial cells can partially correct defects in mouse models of blindness.
One very recent advance—the generation of normal insulin-secreting β islet cells from human iPS and ES cells—shows promise for treatment of both type 1 and type 2 diabetes. Type 1 diabetes results from autoimmune destruction of pancreatic β cells, whereas the more common type 2 diabetes results from insulin resistance in liver and muscle (see Figure 16-40), eventually leading to dysfunction and death of β cells. Patients who receive transplants of human islets from cadavers can be made insulin independent for 5 years or longer, but this approach is limited because of the scarcity and quality of donor islets; thus the possibility of an unlimited supply of human β cells from stem cells could potentially extend this therapy to millions of new patients.
One key to this successful generation of β cells was employing successive treatment with different combinations of growth factors that stimulated iPS or ES cells to traverse the normal embryonic developmental sequence by which the progeny of undifferentiated ICM cells form β cells (Figure 21-9a). The so-called SC-β cells that resulted have a structure very similar to that of normal β islet cells, including secretory granules filled with almost crystalline insulin (see Figure 14-23); they also secrete normal amounts of insulin in response to elevation of the glucose level in their culture medium. Shortly after their transplantation into mice, these cells secrete human insulin into the serum in a glucose-regulated manner. Most important, after transplantation of these cells into immunocompromised diabetic mice, their high glucose levels are lowered to normal (Figure 21-9b), indicating the potential use of these islet cells—which can be produced in culture in essentially unlimited numbers—for the treatment of diabetes. Screening to identify new drugs that improve β cell function, survival, or proliferation can also make use of such a uniform supply of stem cell–derived β cells.
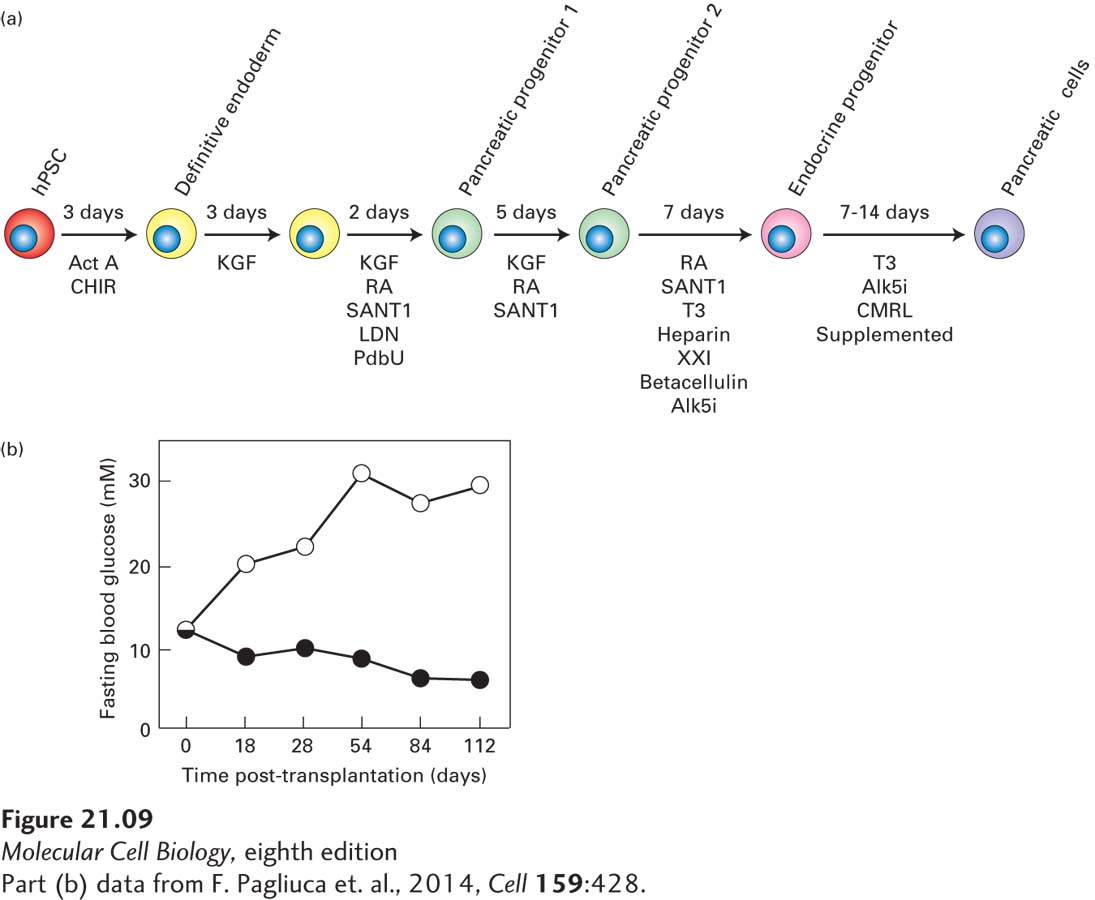
FIGURE 21-9 Production of normal insulin-secreting β islet cells from human iPS or ES cells. (a) Schematic of directed differentiation of human ES or iPS cells into insulin-secreting β islet cells. Clusters of a few hundred human ES or iPS cells were sequentially cultured in media containing the indicated growth factors for the indicated number of days to first produce definitive endoderm cells, then a series of pancreatic progenitor cells, then pancreatic endocrine progenitors, and finally stem cell–derived insulin-producing β islet cells (termed SC-β cells). Act A, activin A; CHIR, GSK3 inhibitor; KGF, keratinocyte growth factor; RA, retinoic acid; SANT1, Sonic Hedgehog pathway antagonist; LDN, a BMP type 1 receptor inhibitor; PdbU, a protein kinase C activator; Alk5i, Alk5 receptor inhibitor II; T3, triiodothyronine, a thyroid hormone; XXI, γ-secretase inhibitor; betacellulin, an EGF family member. (b) SC-β cells can be used to treat diabetes in mice. These experiments used a strain of diabetic mice with a mutation in the insulin gene as well as mutations in several immune-system genes such that the animals did not reject transplants of human tissue. Previous work had shown that the elevated glucose levels in these mice could be restored to normal by transplantation with human pancreatic islets. In this experiment, mice were transplanted with SC-β cells (black circles) or a similar number of control pancreatic progenitor cells (white circles). At the start of the experiment, the average blood glucose level in these mice was about 11 mM, well above the normal 5 mM. The average blood glucose level in the control mice rose continuously to about 30 mM, indicating severe diabetes, while in the mice transplanted with the human SC-β cells, blood glucose dropped to nearly the normal 5 mM.
[Part (b) data from F. Pagliuca et al., 2014, Cell 159:428.]
These SC-β cells are assuredly a harbinger of what is to come. The coming years are certain to see the development of many other types of differentiated cells from human iPS cells that can be used as “replacement parts” for a variety of maladies. Many important questions must be answered, however, before the feasibility of using human ES or iPS cells for therapeutic purposes can be assessed adequately. For instance, when undifferentiated human or mouse ES or iPS cells are transplanted into an experimental mouse, they form teratomas, tumors that contains masses of partially differentiated cell types. Thus it is essential to ensure that all of the ES or iPS cells used to generate an implant have indeed undergone differentiation and have lost their pluripotency and their ability to induce teratomas or cause other problems.
