Germ-Line Stem Cells Produce Sperm or Oocytes
The germ line is the cell lineage that produces oocytes and sperm. It is distinct from the somatic cells that make all the other tissues but are not passed on to progeny. The germ line, like somatic-cell lineages, starts with stem cells, but these cells are unipotent in that they make only germ cells. Stem-cell niches have been especially well defined in studies of germ-line stem cells (GSCs) in Drosophila and C. elegans. Germ-line stem cells are present in adult flies and worms, and the locations of these stem cells are well known.
In the fly, the niche where oocyte precursors form and begin to differentiate is located next to the tip of the germarium, the part of the ovary where eggs are formed (Figure 21-12a). There are two or three germ-line stem cells in this location next to a few cap cells, which create the niche by secreting two proteins in the TGF-β family, Dpp and Gbb, as well as Hedgehog (Hh) protein (Figure 21-12b). (These secreted protein signals were introduced in Chapter 16.) The cap cells create the niche because the TGF-β-class signals they send repress transcription of a key differentiation factor, the Bag of marbles (Bam) protein, in the neighboring germ-line stem cells. Repression of the bam gene allows germ-line stem cells to undergo self-renewing divisions, whereas activation of bam promotes differentiation. When a germ-line stem cell divides, one of the resulting daughters remains adjacent to the cap cells and is therefore maintained as a stem cell, like the parent cell. The other daughter is too far from the cap cells to receive the cap-cell-derived signals Dpp and Gbb. As a result, Bam expression turns on, causing that daughter cell to enter the differentiation program. The signals involved were identified in part through the power of Drosophila genetics: mutant germ-line stem cells with defects in their Dpp or Gbb receptors, or their downstream signal transduction proteins, are lost prematurely. Conversely, overexpression of Dpp by cap cells prevents differentiation of germ-line stem cells and causes formation of tumorlike cell masses.
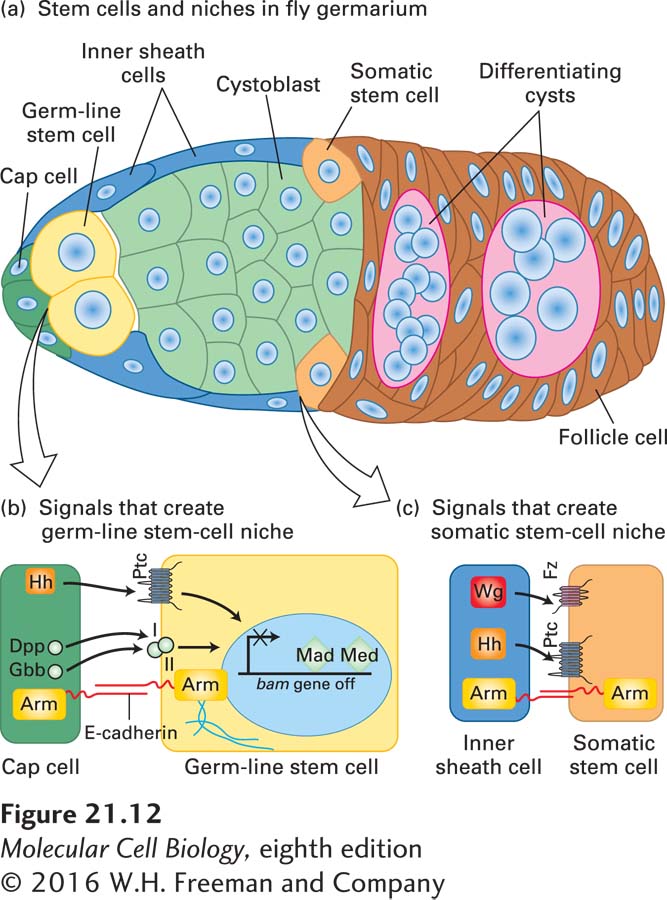
FIGURE 21-12 A Drosophila germarium. (a) Cross section of the germarium, showing female germ-line stem cells (yellow) and some somatic stem cells (gold) in their niches and the progeny cells derived from them. The germ-line stem cells produce cystoblasts (green), which undergo four rounds of mitotic division to produce 16 interconnected cells, one of which becomes the oocyte; the somatic stem cells produce follicle cells (brown), which will make the eggshell. The cap cells (dark green) create and maintain the niche for germ-line stem cells, while the inner sheath cells (blue) produce the niche for somatic stem cells. (b) Signaling pathways that control the properties of germ-line stem cells. The signaling molecules—the TGF-β-family proteins Dpp and Gbb as well as Hedgehog (Hh)—are produced by the cap cells. Binding of these ligands to receptors on the surface of a germ-line stem cell—the TGF-β receptors I and II and Ptc, respectively—results in repression of the bam gene by two transcription factors, Mad and Med. Repression of bam allows germ-line stem cells to self-renew, whereas activation of bam promotes differentiation. The transmembrane cell-adhesion protein E-cadherin forms the homotypic adherens junctions between germ-line stem cells and cap cells. Arm (Armadillo), the fly β-catenin, connects the cytoplasmic tails of the E-cadherin to the actin cytoskeleton; both E-cadherin and Arm are important in maintaining the stem-cell niche. (c) Signaling pathways that control the properties of somatic stem cells. The Wnt signal Wingless (Wg) is produced by the inner sheath cells and is received by the Frizzled receptor (Fz) on a somatic stem cell. Hh is similarly produced and is received by the Ptc receptor. Both of these signals result in self-renewal of somatic stem cells. See L. Li and T. Xie, 2005, Annu. Rev. Cell Devel. Biol. 21:605 and T. Xie, 2013, WIREs Dev. Biol. 2:261.
The stem cells are held in the niche by the transmembrane cell-surface protein E-cadherin (see Chapter 20), which forms adherens junctions via homotypic interactions with similar E-cadherin molecules on the cap cell. These adherens junctions orient the mitotic spindle of the germ-line stem cells such that one daughter remains attached to the cap cell and the other is displaced from the niche; similar asymmetric stem-cell divisions occur during other developmental stages in Drosophila, as we discuss later (see Figures 21-30 and 21-31). Armadillo (Arm), the fly β-catenin, connects the cytoplasmic tails of the E-cadherin molecules to the actin cytoskeleton; like E-cadherin, Arm is important in maintaining the stem-cell niche.
Separate somatic stem cells in the germarium produce follicle cells that will make the eggshell. The somatic stem cells have a niche too, created by the inner sheath cells, which produce Wingless (Wg) protein—a fly Wnt signal—and Hh protein (Figure 21-12c). Hedgehog produced by the cap cells may also play a role. Thus two different populations of stem cells can work in close coordination to produce different parts of an egg.
The identification and characterization of Drosophila germ-line stem cells, as well as similar cells from C. elegans, were important because they convincingly demonstrated the existence of stem-cell niches and permitted experiments to identify the niche-made signals that cause cells to become and remain self-renewing stem cells. Thus a stem-cell niche is a set of cells and the signals they produce, not just a location.
