Rare Types of Cells Constitute the Niche for Hematopoietic Stem Cells
Like all stem cells, hematopoietic stem cells are found in niches. During late embryonic development, HSCs are found in the fetal liver, and in adults, most are localized to the bone marrow. But identifying HSCs and the cells that make up the HSC niche was very complicated. The frequency of HSCs is about 1 per 104 bone marrow or fetal liver cells. Furthermore, identifying HSCs by any technique other than transplantation assays was difficult because no cell-surface protein is expressed only by HSCs, and thus no marker was available for these cells. Much work showed that HSCs could be prospectively identified and purified because they express a cell-surface protein called CD150 (of unknown function) and do not express any of the dozen or so “Lin” (Lineage-restricted) proteins characteristic of other types of hematopoietic progenitor and differentiated cells.
Efforts to identify the niche cells took advantage of the fact that HSCs require a growth factor termed stem cell factor (SCF) for their survival; this protein, which is bound to the surface of an adjacent signaling cell, activates the c-Kit protein tyrosine kinase receptor on HSCs. HSCs also require the secreted protein thrombopoietin, which activates a thrombopoietin receptor that is similar in structure and function to the erythropoietin receptor, and CXCL12, which binds to a G protein–coupled receptor and is required to keep HSCs in the niche. In the fetal liver, only the progenitor cells that generate hepatocytes, the major cell type in the liver, express these three proteins as well as others required for HSC survival. Co-culture of these hepatic progenitor cells with HSCs led to expansion of HSC numbers as well as formation of their differentiated progeny. Thus hepatocyte progenitors were found to be the major cell that forms the HSC niche in the fetal liver.
In adults, a small number of mesenchymal cells surround the small blood vessels, termed sinusoids, that permeate the bone marrow. These cells, called stromal cells, express SCF as well as the receptor for the cytokine leptin on their surface. They also synthesize abundant CXCL12 and are thought to be the major HSC niche cells in the bone marrow (Figure 21-19a). Immunofluorescence analysis showed that about 85 percent of HSCs physically contact these stromal cells (Figure 21-19b). Other cells in the bone marrow probably influence stem-cell maintenance or niche function by releasing other types of hormones.
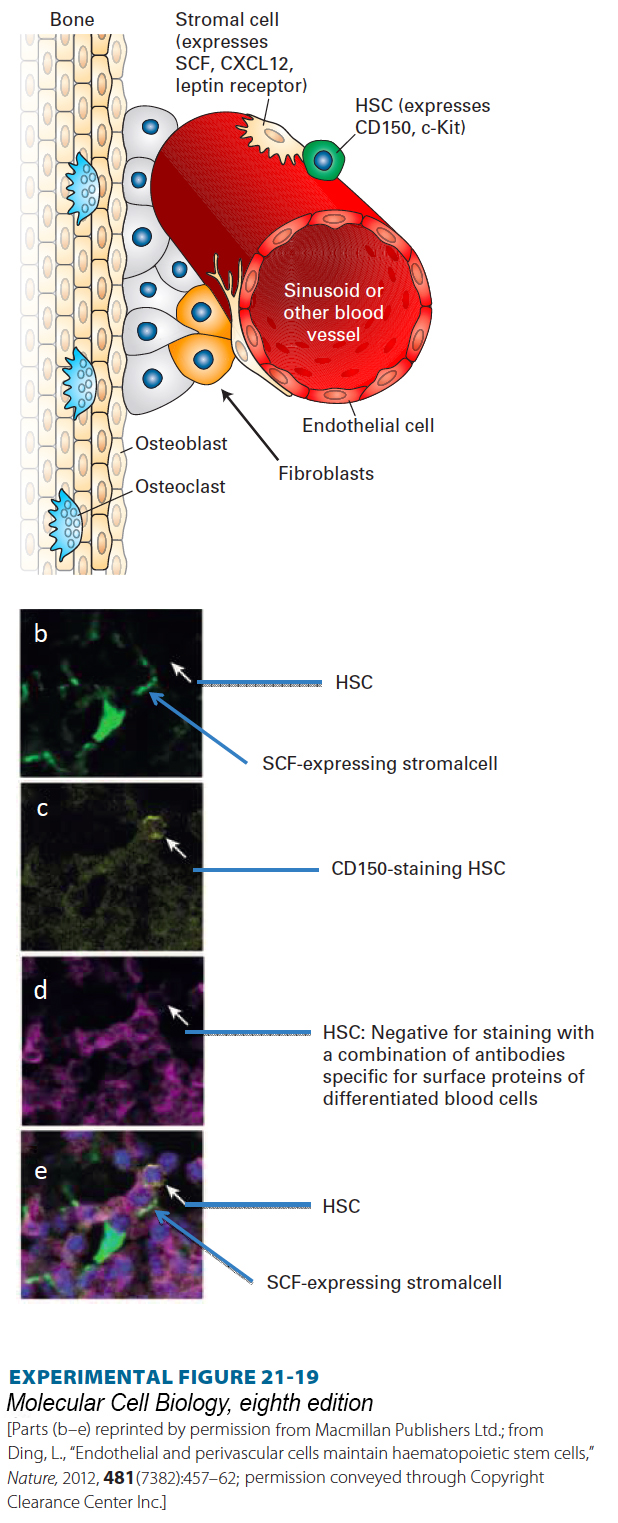
EXPERIMENTAL FIGURE 21-19 The hematopoietic stem-cell niche in the bone marrow. (a) The bone marrow contains dozens of different types of cells, including osteoblasts and osteoclasts that build and degrade bone, respectively, as well as multiple types of hematopoietic cells, fibroblasts, and other cell types. The bone marrow is permeated by small blood vessels termed sinusoids. The predominant cells that form the HSC niche are the very rare mesenchymal stromal cells that adhere to these vessels and that express a combination of cell-surface proteins including SCF, the hormone that binds to and activates the c-Kit protein tyrosine kinase receptor on HSCs. These stromal cells also express the receptor for the cytokine leptin and secrete CXCL12, a chemoattractant for HSCs. See S. Morrison and D. Scadden, 2014, Nature 505:327. (b–d) Immunofluorescence detection of HSCs and niche cells in bone marrow, showing that HSCs are localized next to SCF-expressing cells. Antibodies to SCF were not available, so in order to detect SCF expression, a mouse was generated in which GFP cDNA was placed in the SCF gene locus and expressed only in cells that normally produce SCF. Bone marrow sections were then examined in a fluorescence microscope to detect the SCF-expressing cells (b). To detect HSCs, the sections were stained with an antibody to the CD150 protein, expressed in HSCs (c). The sections were also stained with a collection of antibodies (d) specific for proteins expressed by different types of differentiated blood cells, but not by HSCs. (e) When the three images are merged, the HSC (white arrows) can be seen lying adjacent to the SCF-expressing stromal cell.
[Parts (b–e) reprinted by permission from Macmillan Publishers Ltd.; from Ding, L., “Endothelial and perivascular cells maintain haematopoietic stem cells,” Nature, 2012, 481(7382):457–62; permission conveyed through Copyright Clearance Center Inc.]
You have probably noticed that all the molecular regulators of stem cells that we have discussed are familiar proteins (see Chapters 15 and 16) rather than dedicated regulators that specialize in stem-cell control. Each type of signal is used repeatedly to control cell fates and growth. Stem cells are regulated by ancient signaling systems, at least a half billion years old, for which new uses have emerged as cells, tissues, organs, and animals have evolved new variations.
