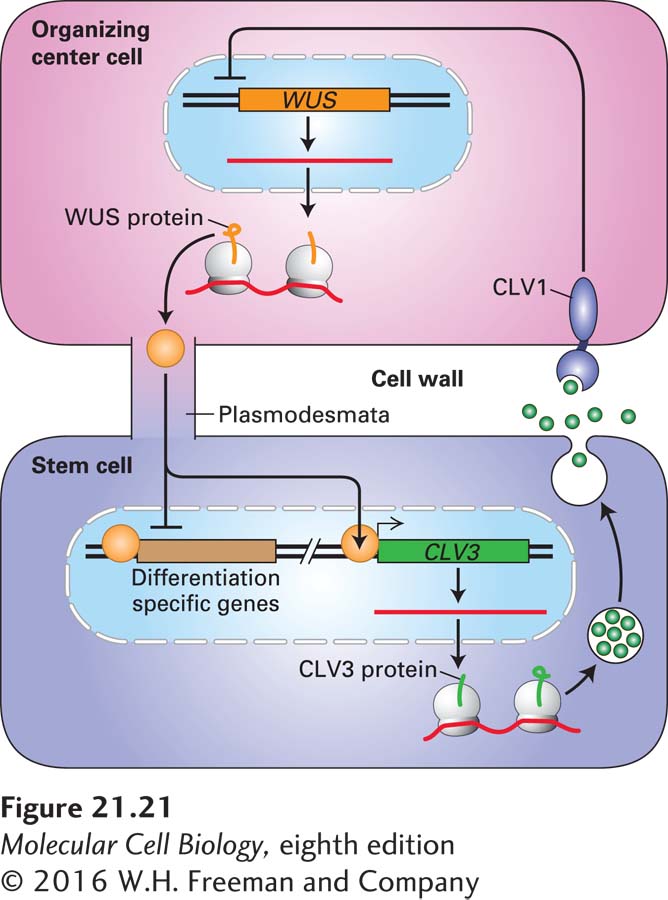
Genes required for stem-cell identity, maintenance, and cell differentiation have been defined by genetic screens in the mustard-family weed Arabidopsis thaliana (see Figure 1-22h) for mutants exhibiting larger, smaller, or non-replenishing meristems as well as by more recent gene-expression profiling studies of isolated meristem-cell populations. One shoot apical meristem determinant is the gene called WUSCHEL (WUS), which encodes a homeodomain transcription factor (see Chapter 9). WUS is required for maintenance of the stem-cell population but is expressed in the supportive cells underlying the stem cells. These cells, collectively termed the organizing center, are analogous to the niche cells in metazoans (see Figure 21-20a). While WUS mRNA and protein is synthesized in the cells of the organizing center, a series of experiments showed that WUS moves from the organizing-center cells into the stem cells, presumably through the interconnecting plasmodesmata (see Figure 20-41). In one study, a WUS-GFP fusion protein, when expressed in WUS-negative Arabidopsis plants, was able to rescue the mutant phenotype. Subsequent microscopic analysis showed that this WUS-GFP chimera accumulated in the stem cells, indicating it had moved there from the organizing-center cells.
Once in the stem cells, WUS binds to many sites in the genome; it represses a large number of genes that are expressed in differentiating cells, including a group of differentiation-promoting transcription factors required for leaf development. WUS also directly activates the expression of CLAVATA3 (CLV3) in stem cells. CLV3 encodes a small secreted peptide that binds to the CLV1 receptor on the surface of organizing-center cells and generates an intracellular signal that negatively regulates WUS expression. Overexpression of WUS causes a large expansion of the meristem stem-cell population at the expense of production of differentiated cells. Thus the negative feedback loop between a transcription factor, WUS, and a signaling peptide, CLV3, maintains the size of the stem-cell population and the number of their dividing daughter cells over the lifetime of the plant (Figure 21-21).