The Intrinsic Polarity Program Depends on a Positive Feedback Loop Involving Cdc42
Budding yeast grows by selecting a single site on its surface at which to assemble a new bud (see Figure 19-3). Importantly, it must reliably select just one site. If a cell grew two buds simultaneously, consider what would happen during mitosis: the duplicated chromosomes might be segregated between the parent cell and one bud, leaving the other bud without chromosomes and therefore inviable. In haploid yeast, this so-called singularity of budding is guided by a signal, or cue, left at the cell surface, which directs the next budding event to a site adjacent to the former budding site. Remarkably, if the genes that specify the nonessential components of this cue are deleted, yeast cells grow just as well, but each assembles a single bud at a random site. This result reveals that yeast has an intrinsic polarity program that, even in the absence of cues from the previous budding cycle, can guide selection of a single site for bud growth. This program requires the concentration of Cdc42 at the site from which a bud will emerge.
Surprisingly, Cdc42 concentration at the site for a new bud does not depend on either actin filaments or microtubules, as this small GTPase localizes to a single spot even when both filament systems are disrupted (Figure 21-22a). Long before biologists had thought about how this might occur, the brilliant mathematician and computer pioneer Alan Turing considered what mechanism might shift a uniform distribution of a polarity factor to a concentration at a single site. In 1952, Turing suggested that such a shift could be achieved if a positive feedback reaction amplified a random increase in the concentration of the polarity factor—and he was right!
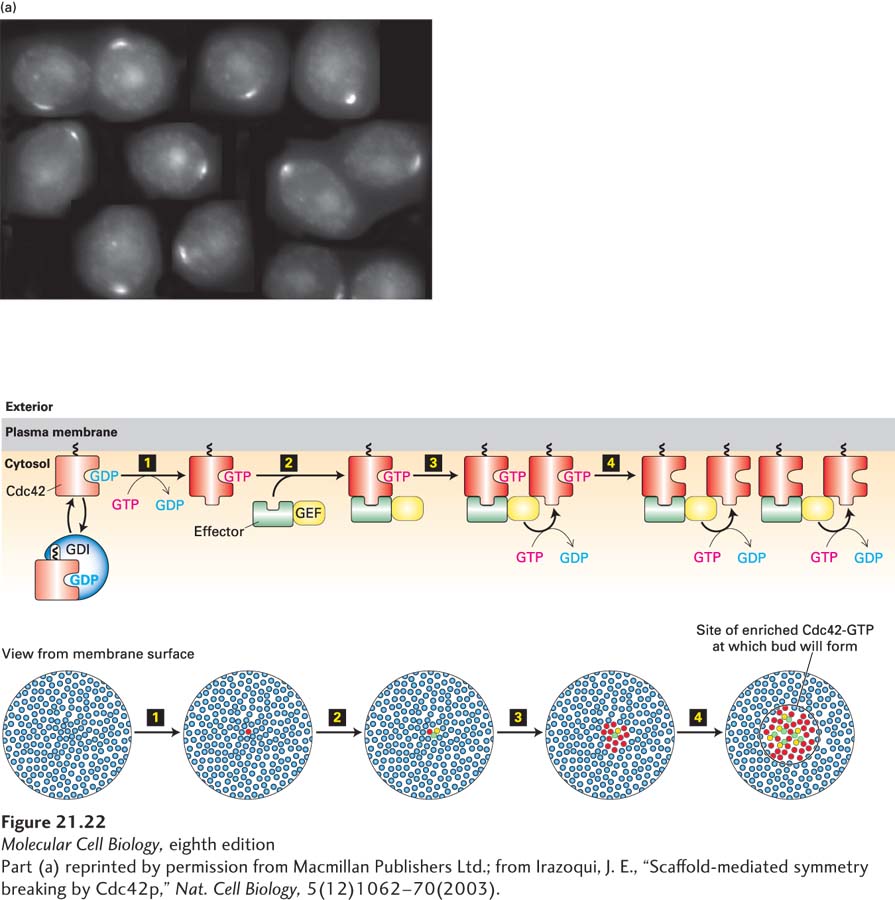
FIGURE 21-22 The intrinsic polarity program of budding yeast involves a positive feedback loop for activation of the GTPase Cdc42. (a) Diploid yeast lacking polarity cues show polarized Cdc42, visualized here by immunofluorescence microscopy, when they are about to assemble a bud. The cells were treated with drugs to disassemble both actin filaments and microtubules to show that polarization of Cdc42 is not dependent on these cytoskeletal filaments. (b) Positive feedback loop for activation of Cdc42. Inactive Cdc42·GDP is in equilibrium between a cytosolic pool of complexes with the guanine nucleotide dissociation inhibitor (GDI) and a membrane-bound pool. Step 1: One of the membrane-associated Cdc42·GDP proteins may spontaneously become an activated Cdc42·GTP. Step 2: Active Cdc42·GTP recruits a complex containing the guanine nucleotide exchange factor (GEF). Step 3: The recruited GEF now locally converts more Cdc42·GDP to Cdc42·GTP. Step 4: This active Cdc42·GTP recruits more GEF, thus driving a positive feedback loop that results in the local accumulation of Cdc42·GTP. See C.-F. Wu and D. Lew, 2013, Trends Cell Biol. 23:476.
[Part (a) reprinted by permission from Macmillan Publishers Ltd.; from Irazoqui, J. E., “Scaffold-mediated symmetry breaking by Cdc42p,” Nat. Cell Biology, 5(12)1062–70(2003).]
Recall that Cdc42 is a member of the Rho family of small GTP-binding proteins (see Figure 17-41). It acts as a molecular switch, existing in an inactive (Cdc42·GDP) and an active (Cdc42·GTP) state. Binding to its specific guanine nucleotide exchange factor (Cdc42-GEF) causes Cdc42 to release GDP and bind GTP. The active Cdc42·GTP binds effectors and thereby activates downstream signaling events. In its inactive state, Cdc42·GDP exists in equilibrium between a cytosolic pool, which is bound to a guanine nucleotide dissociation inhibitor (GDI), and a membrane-bound Cdc42·GDP pool (Figure 21-22b). Occasionally and randomly, the membrane-bound Cdc42·GDP will release its GDP and bind GTP, which converts it to the active Cdc42·GTP state (Figure 21-22b, step 1). One of the effectors that is recruited to Cdc42·GTP is a protein complex that contains Cdc42-GEF (step 2). Thus, when an active Cdc42 arises in the plasma membrane, it recruits Cdc42-GEF, which locally activates more Cdc42, which recruits more Cdc42-GEF, and this simple positive feedback loop generates a site highly and locally enriched for Cdc42·GTP on the cell surface (steps 3 and 4). Computational modeling—also pioneered by Turing—shows that this system can result in a “winner-takes-all” scenario to yield just one site of polarization. This positive feedback loop is the core mechanism by which one very stable budding site is generated in yeast.
As we saw in Chapter 18, Cdc42 is also the master regulator that guides the polarization of migrating cells (see Figure 18-53), in which a similar type of feedback cycle also probably exists. As we will see, Cdc42 is involved in regulating many additional examples of cell polarity. It is important to note that in cases in which polarization needs to be more flexible, negative feedback loops ensure that the single site of polarization is not too strong, so that it can be redirected to another site on the cell surface upon receiving appropriate signals. For example, in addition to its fast-acting GEF, Cdc42·GTP might also recruit a slow-acting negative regulator that modulates the degree of positive feedback. In fact, yeast Cdc42 recruits a kinase that phosphorylates and inhibits the recruited Cdc42-GEF, thereby introducing a negative feedback loop. Thus the local concentration of Cdc42·GTP builds up fast, then levels off or disappears as the slower negative regulator comes into play. The biochemical basis of these positive and negative feedback loops is an active and important area of current research. As we will see, specific cues normally guide these intrinsic polarity programs, which in turn lead to the physical polarization of the cell.
