Neurotrophins Promote Survival of Neurons
In mammals, but not in nematodes, apoptosis is regulated by intracellular signals generated from many secreted and cell-surface protein hormones, as well as by many environmental stresses, such as ultraviolet irradiation and DNA damage. While the “core” apoptosis machinery in C. elegans is conserved in mammals, many other intracellular proteins also regulate apoptosis (see Figure 21-35, right).
But before plunging into these molecular details, we’ll illustrate the importance of trophic factors in apoptosis with a brief analysis of the developing nervous system. When neurons grow to make connections to other neurons or to muscles, sometimes over considerable distances, more neurons grow than will eventually survive. The cell bodies of many sensory and motor neurons are located in the spinal cord and adjacent ganglia, while their long axons extend far outside these regions. Those neurons that make signaling connections, termed synapses (see Figure 22-3), with their intended target cells prevail and survive; those that fail to connect will die.
In the early 1900s, the number of neurons innervating peripheral cells was shown to depend on the size of the tissue to which they connect, the so-called target field. For instance, removal of limb buds from a developing chick embryo leads to a reduction in the number of both sensory and motor neurons innervating muscles in the bud (Figure 21-37). Conversely, grafting additional limb tissue to a limb bud leads to an increased number of neurons in the corresponding regions of the spinal cord and sensory ganglia. Indeed, incremental increases in target-field size are accompanied by commensurate incremental increases in the number of neurons innervating the target field. This relationship was found to result from the selective survival of neurons, rather than changes in their differentiation or proliferation. The observation that many sensory and motor neurons die after reaching their peripheral target field suggested that these neurons compete for survival factors produced by the target tissue.
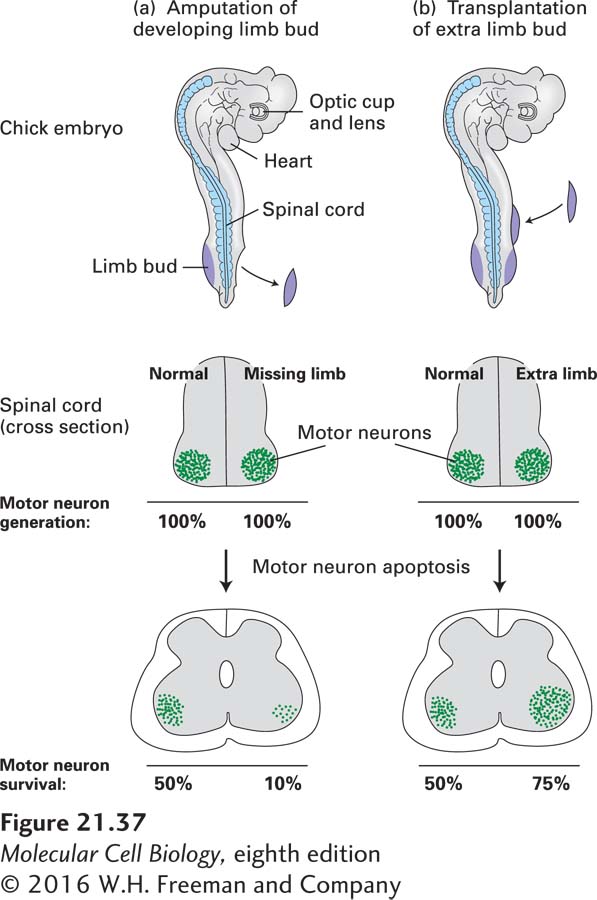
EXPERIMENTAL FIGURE 21-37 In vertebrates, the survival of motor neurons depends on the size of the muscle target field they innervate. (a) Removal of a limb bud from one side of a chick embryo at about 2.5 days results in a marked decrease in the number of motor neurons on the affected side. In an amputated embryo (top), normal numbers of motor neurons are generated on both sides (middle). Later in development, however, many fewer motor neurons remain on the side of the spinal cord with the missing limb than on the normal side (bottom). Note that only about 50 percent of the motor neurons that are generated normally survive. (b) Transplantation of an extra limb bud into an early chick embryo produces the opposite effect, more motor neurons on the side with additional target tissue than on the normal side. See D. Purves, 1988, Body and Brain: A Trophic Theory of Neural Connections, Harvard University Press, and E. R. Kandel, J. H. Schwartz, and T. M. Jessell, 2000, Principles of Neural Science, 4th ed., McGraw-Hill, p. 1054, Figure 53-11.
Subsequent to these early observations, scientists discovered that transplantation of a mouse sarcoma (muscle tumor) into a chick led to a marked increase in the local numbers of certain types of neurons. This finding implicated the tumor as a rich source of a presumed trophic factor. To isolate and purify this factor, known simply as nerve growth factor (NGF), scientists used cell culture assays in which outgrowth of neurites from sensory ganglia was measured. Neurites are extensions of the neuronal cytoplasm that can grow to become the long processes of the nervous system, the axons and dendrites (see Figure 22-1). The later discovery that the submaxillary gland of the mouse also produces large quantities of NGF enabled Rita Levi-Montalcini to purify and sequence it; she was rewarded with a Nobel Prize. A homodimer of two 118-residue polypeptides, NGF belongs to a family of structurally and functionally related trophic factors collectively referred to as neurotrophins. Brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) are also members of this protein family.
Neurotrophins bind to and activate a family of receptor tyrosine kinases called Trks (pronounced “tracks”). (The general structure of receptor tyrosine kinases and the intracellular signaling pathways they activate are covered in Chapter 16.) Each neurotrophin binds with high affinity to one type of Trk receptor: NGF binds to TrkA; BDNF, to TrkB; and NT-3, to TrkC. NT-3 can also bind with lower affinity to both TrkA and TrkB. All neurotrophins also bind to a distinct type of receptor called p75NTR (also called NTR 5 neurotrophin receptor), but with lower affinity; p75NTR forms heteromeric complexes with the different Trk receptors. These binding relationships between trophic factors and their receptors provide survival signals for different classes of neurons. As nerve exons extend outward from the spinal cord to the periphery, neurotrophins produced by their target tissues bind to Trk receptors on the growth cones (see Figure 18-54) at the tips of the extending axons, promoting survival of those neurons that successfully reach their targets.
To investigate the role of neurotrophins in development, scientists produced mice with knockout mutations in each of the neurotrophins or their receptors. These studies revealed that different neurotrophins and their corresponding receptors are required for the survival of different classes of sensory neurons (Figure 21-38). For instance, pain-sensitive (nociceptive) neurons, which express TrkA, are selectively lost from the dorsal root ganglion of knockout mice lacking NGF or TrkA, whereas TrkB- and TrkC-expressing neurons are unaffected in such knockouts. In contrast, TrkC-expressing proprioceptive neurons, which detect the position of the limbs, are missing from the dorsal root ganglion in TrkC and NT-3 mutants.
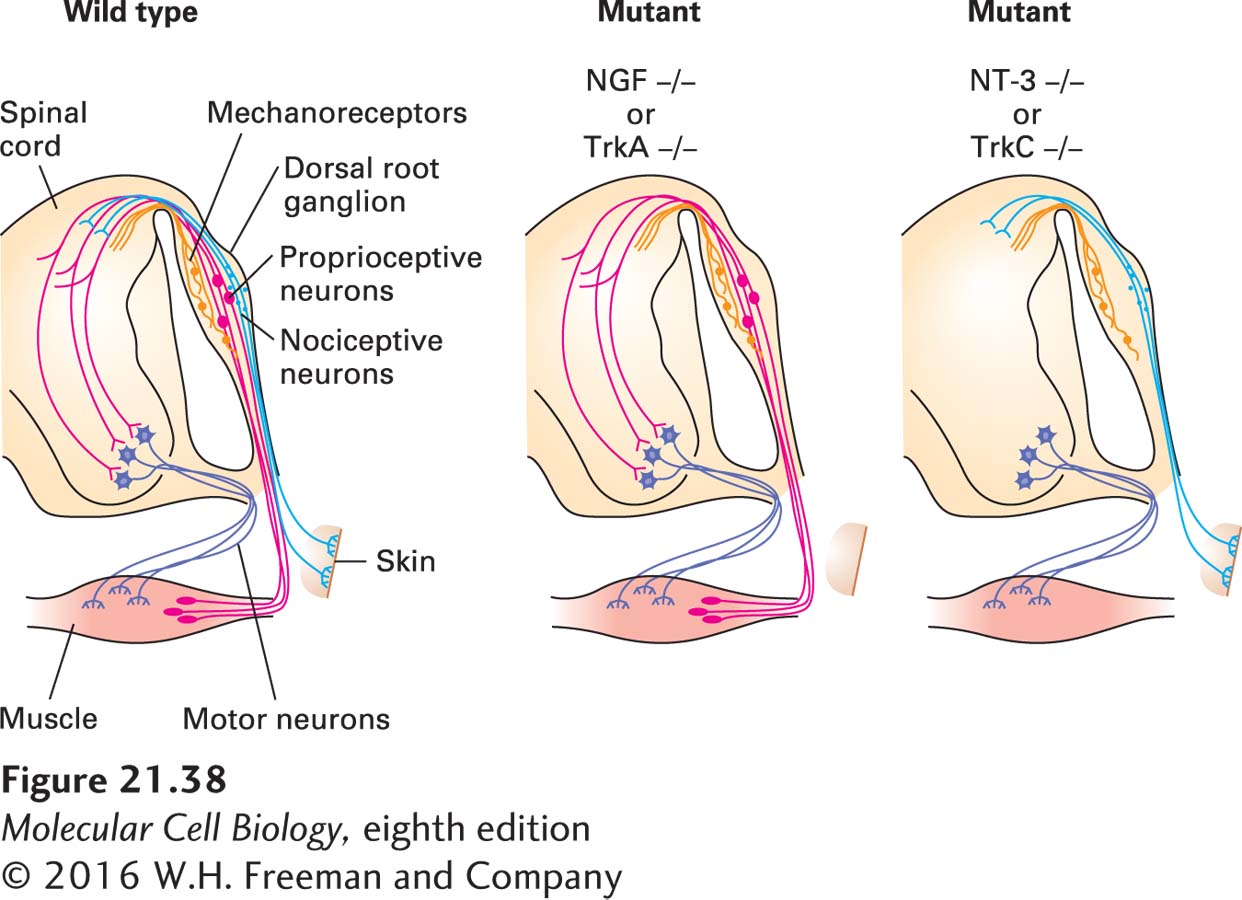
EXPERIMENTAL FIGURE 21-38 Different classes of sensory neurons are lost in knockout mice lacking different trophic factors or their receptors. In animals lacking nerve growth factor (NGF) or its receptor TrkA, small nociceptive (pain-sensing) neurons (light blue) that innervate the skin are missing. These neurons express the TrkA receptor and innervate NGF-producing target tissues. In animals lacking either neurotrophin-3 (NT-3) or its receptor TrkC, large proprioceptive neurons (red) innervating muscle spindles are missing. Muscle tissue produces NT-3, and the proprioceptive neurons express TrkC. Mechanoreceptors (orange; see Figure 22-32), another class of sensory neurons in the dorsal root ganglion, are unaffected in these mutants. See W. D. Snider, 1994, Cell 77:627.

