Release of Cytochrome c and SMAC/DIABLO Proteins from Mitochondria Leads to Formation of the Apoptosome and Caspase Activation
The principal way in which cytochrome c in the cytosol activates apoptosis is by binding Apaf-1, the mammalian homolog of CED-4 (see Figure 21-35, right). In the absence of cytochrome c, monomeric Apaf-1 is bound to dATP. After binding cytochrome c, Apaf-1 cleaves its bound dATP into dADP and Pi and undergoes a dramatic assembly into a disk-shaped heptamer, a 1.4-MDa wheel of death called the apoptosome (Figure 21-41). The apoptosome serves as an activation machine for the initiator caspase caspase-9, which is monomeric in the inactive state. Initiator caspases must be sensitive to activation signals, yet should not be activatable in an irreversible manner because accidental activation would lead to an undesirable snowball effect and rapid cell death. Significantly, caspase-9 does not require cleavage to become activated, but rather is activated by dimerization following binding to the apoptosome. Caspase-9 then cleaves multiple molecules of effector caspases, such as caspase-3 (see Figures 21-35 and 21-40), leading to their activation and subsequent destruction of cell proteins and cell death. The three-dimensional structure of the corresponding nematode CED-4 apoptosome (Figure 21-41c) shows how two CED-3 procaspases bind adjacent to each other on the inside of the funnel-shaped octamer; these molecules then activate each other by dimerization and proteolytic conversion. The structure of the CED-4 apoptosome also provides a model for the as yet unknown three-dimensional structure of the corresponding mammalian apoptosome (Figure 21-41b, right).
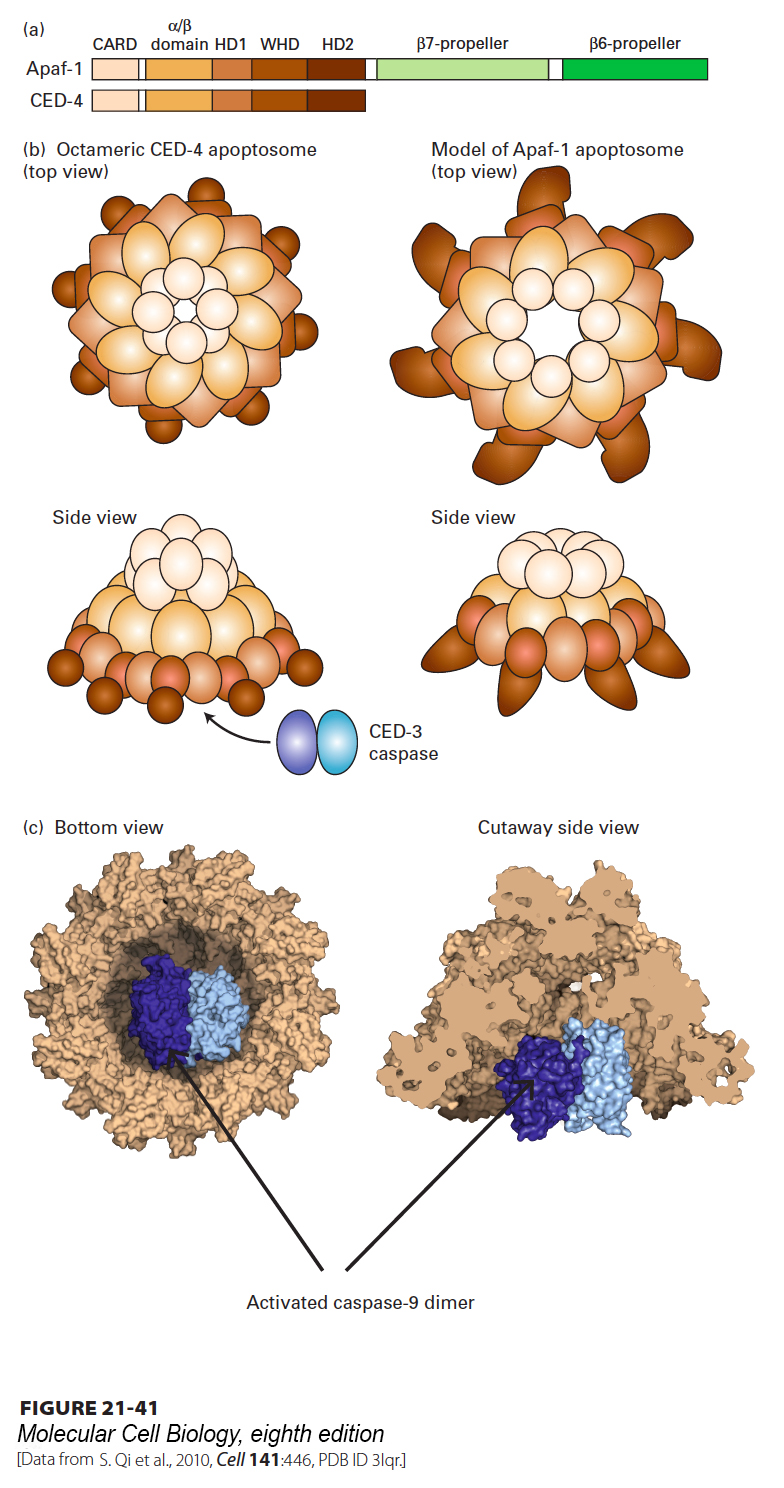
FIGURE 21-41 Structure of the nematode apoptosome and a model for the structure of the mammalian Apaf-1 apoptosome. (a) Domains of the CED-4 protein and the corresponding mammalian Apaf-1; CARD stands for N-terminal caspase recruitment domain. In the oligomeric apoptosome, these CARD domains bind to CARD domains in the caspases. (b) Diagram of the CED-4 apoptosome (left) and a model for the corresponding mammalian apoptosome (right). (c) Three-dimensional structure of the nematode octameric CED-4 apoptosome, showing the binding of two CED-3 procaspases. Interaction of the apoptosome with CED-3 stimulates CED-3 dimerization, which is necessary for its activation.
[Data from S. Qi et al., 2010, Cell 141:446, PDB ID 3lqr.]
In mammals and flies, but not in nematodes, apoptosis is regulated by several other proteins (see Figure 21-35, right). XIAP, one member of a family of inhibitor of apoptosis proteins (IAPs), provides another way to restrain both initiator and effector caspases. XIAP has three N-terminal BIR domains; the one termed BIR2 binds to and inhibits two effector caspases, caspase-3 and caspase-7, while BIR3 binds to and inhibits initiator caspase-9. (Other members of the IAP family inhibit apoptosis induced by TNFα; see Figure 21-42 below.) The inhibition of caspases by IAPs, however, creates a problem when a cell needs to undergo apoptosis. Mitochondria enter the picture once again, since they are the source of a family of proteins, called SMAC/DIABLOs, that inhibit IAPs. Assembly of Bax or Bak oligomers (see Figure 21-40) leads to the release of SMAC/DIABLOs, as well as cytochrome c, from mitochondria. SMAC/DIABLOs then bind to XIAP in the cytosol, thereby blocking XIAP from binding to caspases. By relieving XIAP-mediated inhibition, SMAC/DIABLOs promote caspase activity and cell death.
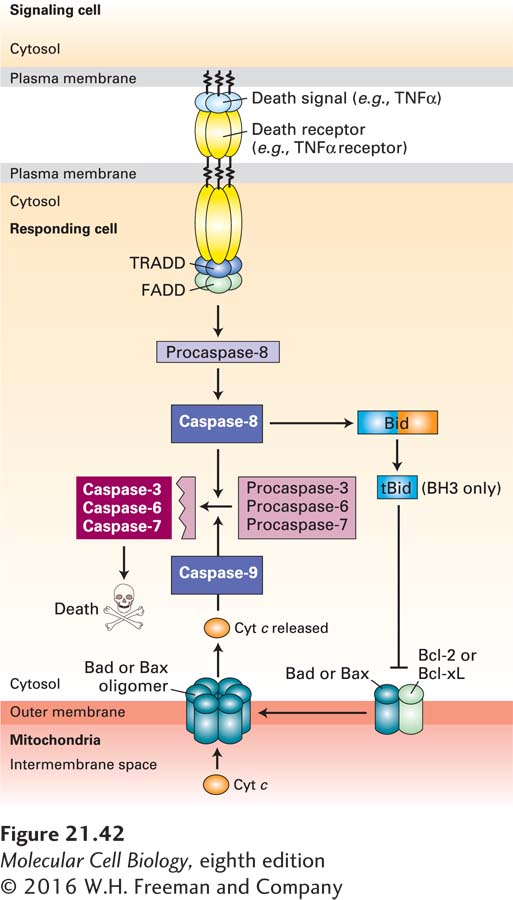
FIGURE 21-42 Cell murder: the extrinsic apoptosis pathway. Extrinsic (or death receptor–regulated) apoptosis pathways are found in many types of cells. In this example, binding of TNFα on the surface of one cell to the TNFα death receptor on an adjacent cell leads to recruitment of the adapter proteins TRADD (TNF receptor-associated death domain protein) and FADD (Fas-associated death domain protein) and the dimerization and activation of the initiator caspase-8. Active caspase-8 then cleaves and activates effector caspases-3, -6, and -7, which cleave vital cellular proteins and induce cell death. Cleavage of the BH3-only protein Bid (BH3-interacting-domain death agonist) by caspase-8 generates a tBid fragment that binds to Bcl-2 on the outer mitochondrial membrane, leading to release of cytochrome c into the cytosol and activation of the intrinsic apoptosis pathway (see Figure 21-39) as well. See P. Bouillet and L. O’Reilly, 2009, Nat. Rev. Immunol. 9:514, and A. Ashkenazi and G. Salvesen, 2014, Annu. Rev. Cell Dev. Biol. 30:20.

