All Voltage-Gated Ion Channels Have Similar Structures
Having explained how the action potential is dependent on regulated opening and closing of voltage-gated channels, we turn to a molecular dissection of these remarkable proteins. After describing the basic structure of these channels, we focus on three questions:
How do these proteins sense changes in membrane potential?
How is this change transduced into opening of the channel?
What causes these channels to become inactivated shortly after opening?
The initial breakthrough in understanding voltage-gated ion channels came from analysis of fruit flies (Drosophila melanogaster) carrying the shaker mutation. These flies shake vigorously under ether anesthesia, reflecting a loss of motor control and a defect in certain motor neurons that have an abnormally prolonged action potential. Researchers suspected that the shaker mutation caused a defect in channel function. Cloning of the gene involved confirmed that the defective protein was a voltage-gated K+ channel. The shaker mutation prevents the mutant channel from opening normally immediately upon depolarization. To establish that the wild-type shaker gene encoded a K+ channel, cloned wild-type shaker cDNA was used as a template to produce shaker mRNA in a cell-free system. Expression of this mRNA in frog oocytes and patch-clamp measurements on the newly synthesized channel protein showed that its functional properties were identical with those of the voltage-gated K+ channel in the neuronal membrane, demonstrating conclusively that the shaker gene encodes this K+-channel protein.
The Shaker K+ channel and most other voltage-gated K+ channels that have been identified are tetrameric proteins composed of four identical subunits arranged in the membrane around a central pore. Each subunit is constructed of six membrane-spanning α helices, designated S1–S6, and a P segment (Figure 22-13a). The S5 and S6 helices and the P segment are structurally and functionally homologous to those in the nongated resting K+ channel discussed earlier (see Figure 11-20); the S5 and S6 helices form the lining of the K+ selectivity filter through which the ion travels. The S1–S4 helices form a rigid complex that functions as a voltage sensor (with positively charged side chains in S4 acting as the primary sensor). The N-terminal “ball” extending into the cytosol from S1 is the channel-inactivating segment.
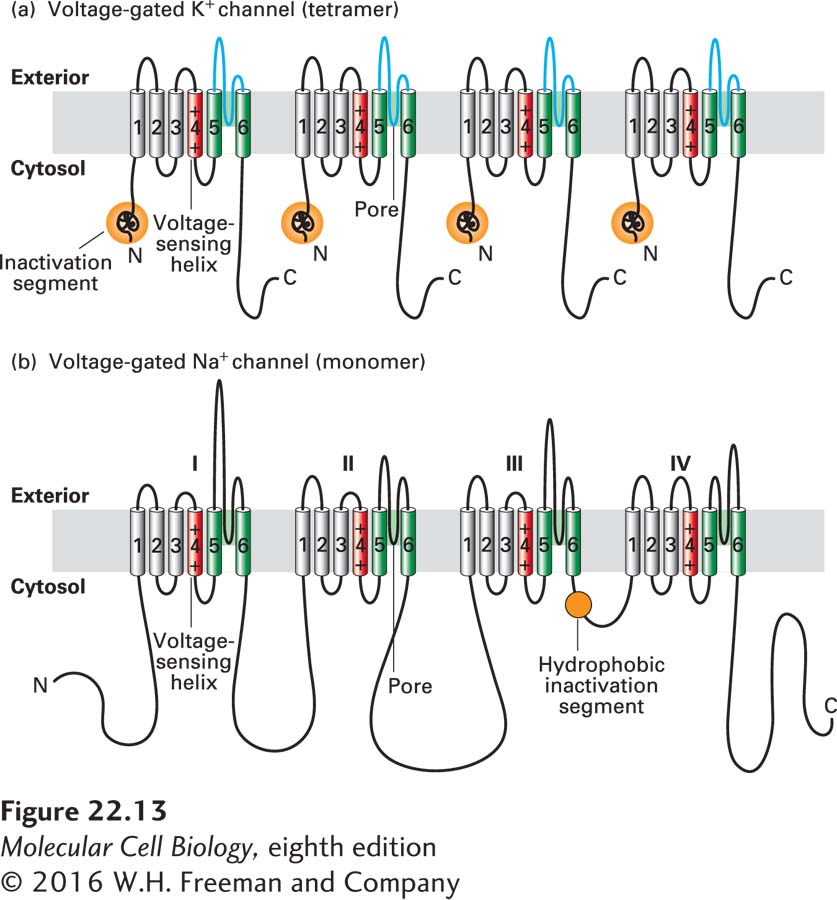
FIGURE 22-13 Schematic depictions of the secondary structures of voltage-gated K+ and Na+ channels. (a) Voltage-gated K+ channels are composed of four identical subunits, each containing 600–700 amino acids, and six membrane-spanning α helices, S1–S6. The N-terminus of each subunit, located in the cytosol and labeled N, forms a globular domain (orange ball) essential for inactivation of the open channel. The S5 and S6 helices (green) and the P segment (blue) are homologous to those in nongated resting K+ channels, but each subunit contains four additional transmembrane α helices. One of these, S4 (red), is the primary voltage-sensing α helix and is assisted in this role by forming a stable complex with helices S1–S3. See C. Miller, 1992, Curr. Biol. 2:573, and H. Larsson et al., 1996, Neuron 16:387. (b) Voltage-gated Na+ channels are monomers containing 1800–2000 amino acids organized into four transmembrane domains (I–IV) that are similar to the subunits in voltage-gated K+ channels. The single hydrophobic channel-inactivating segment (orange ball) is located in the cytosol between domains III and IV. Voltage-gated Ca2+ channels have a similar overall structure. Most voltage-gated ion channels also contain regulatory (β) subunits, which are not depicted here. See W. A. Catterall, 2001, Nature 409:988.
Voltage-gated Na+ channels are monomeric proteins organized into four homologous domains, I–IV (Figure 22-13b). Each of these domains is similar to a subunit of a voltage-gated K+ channel. However, in contrast to voltage-gated K+ channels, which have four channel-inactivating segments, the monomeric voltage-gated channels have a single channel-inactivating segment. Except for this minor structural difference and their varying ion permeabilities, all voltage-gated ion channels are thought to function in a similar manner and to have evolved from a monomeric ancestral channel protein that contained six transmembrane α helices. The next section will focus on the voltage-gated K+ channels, since the crystal structures of both prokaryotic and eukaryotic K+ channels were solved over a decade ago, with subsequent studies refining our understanding of the structural basis of their function. We will also, however, compare and contrast this structure with that of prokaryotic voltage-gated Na+ channels, whose molecular structure was solved in 2011.
