Light-Activated Ion Channels and Optogenetics
Unicellular flagellates such as Chlamydomonas (Figure 1-22b) respond rapidly to light through phototaxis, which guides them toward the light, or photophobia, which prevents them from moving toward the light. These rapid photomotility responses are mediated by light-activated cation channels that reside in a specialized photoreceptive organelle called the eyespot. The first light-activated channel to be cloned was from the green algae Chlamydomonas reinhardtii. This channel, called channelrhodopsin, consists of a seven-transmembrane protein covalently linked to a photo-isomerizable chromophore, all-trans-retinal (Figure 22-19a, b). This is the same chromophore that is used to detect photons in the eye, as discussed in Chapter 15 (see Figure 15-22). When all-trans-retinal absorbs a photon, it converts to 13-cis-retinal, which induces a conformational change in the channel protein, leading to the opening of a pore with a diameter of approximately 0.6 nm and the rapid influx of cations. Channelrhodopsins are nonselective cation channels that conduct H+, Na+, K+, and Ca2+ ions. Many other light-activated cation channels have now been identified, and genetic engineering approaches have generated a series of designer channelrhodopsins that are activated or inactivated by specific wavelengths and intensities of light, and to be selective for distinct cations. These advances have given rise to the exciting new field of optogenetics, in which channelrhodopsins are expressed in electrically excitable cells, permitting the use of light to rapidly and selectively manipulate the membrane potential of the cell (Figure 22-19c).
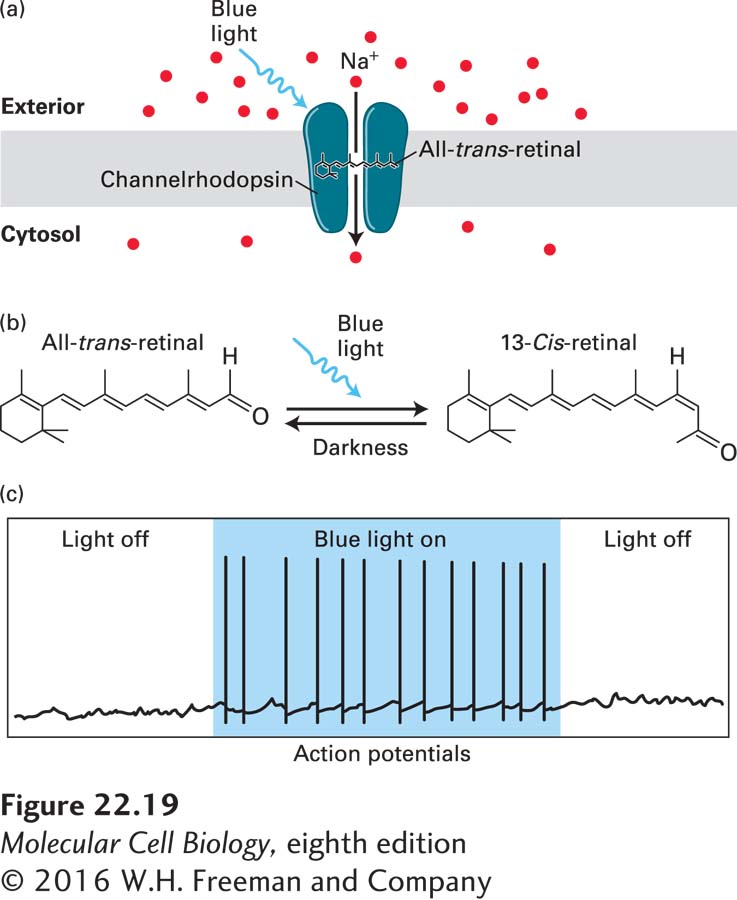
FIGURE 22-19 Channelrhodopsins and optogenetics: activating neurons with light. (a) Light-activated ion channels. Channelrhodopsins are light-activated cation channels that were initially isolated from the green algae Chlamydomonas reinhardtii, where they mediate phototaxic responses. The channel has seven transmembrane domains, and is covalently linked to the photo-isomerizable chromophore all-trans-retinal. (b) All-trans-retinal absorbs blue light (~470 nm) and changes to the 13-cis-retinal conformation. This leads to a conformational change in the channel, opening a pore through which cations can flow. Opening of channelrhodopsin, shown in (a), leads to influx of Na+ from the extracellular solution into the cell, resulting in rapid depolarization of excitable cells. When the light is removed, retinal returns to the all-trans conformation and the channel closes. This is shown in (c) where illumination of a neuron expressing channelrhodopsin with blue light triggers actions potentials as long as the light is on. See J. Wong, O. J. Abilez, and E. Kuhl, 2012, J. Mech. Phys. Solids 60:1158–1178.
Optogenetic approaches have revolutionized the study of neuronal circuits in the brain because they allow neuroscientists to directly test the effect of neural circuit activity on behavior, rather than to simply record neural activity and correlate it with behavior. A common experimental approach is to make transgenic mice in which channelrhodopsin is expressed under the control of a cell-type-specific promoter (described in Chapter 6) so that only a subset of neurons in the brain express the protein. A hole is then made in the skull, and lasers are used to illuminate channelrhodopsin-expressing cells near the surface of the brain, or alternatively a fiber-optic cable is used to deliver light to channelrhodopsin-expressing cells in deeper brain regions. The light triggers action potentials in the neurons, activating their circuits. By examining the behavior of the mouse, one can link the circuit with a specific behavior.
As one example of this type of experiment, the identity of the neurons in the brain that mediate thirst was recently discovered using optogenetics. To do this, channelrhodopsin was expressed in a region of the hypothalamus called the subfornical organ (SFO) in mice (Figure 22-20). In one mouse line, channelrhodopsin was expressed exclusively in excitatory neurons, and in the other mouse line, it was expressed in inhibitory neurons. Light was delivered to the SFO using a fiber-optic cable, and when the excitatory neurons were depolarized with light, the mice exhibited thirst (seeking water and drinking intensely), but when the inhibitory neurons were activated, thirst was suppressed. Additional experiments showed that the drinking response is specific to water, and is not activated by other compounds such as mineral oil or glycerol. Together these experiments demonstrate that neurons in the SFO of the hypothalamus regulate thirst, with activation of excitatory neurons promoting thirst and activation of inhibitory neurons repressing thirst. Similar approaches have been used to uncover circuits underlying many other behaviors, including locomotion, feeding and overeating, anxiety, aggression, and other social behaviors. In addition, optogenetic approaches can be used with other methods, such as calcium imaging (see Chapter 4 and below, Section 22.3) to map out the anatomy of specific circuits in the brain. This is done by activating a specific neuron with light and then using imaging approaches to visualize activity (e.g., by monitoring calcium dynamics) in downstream neurons.
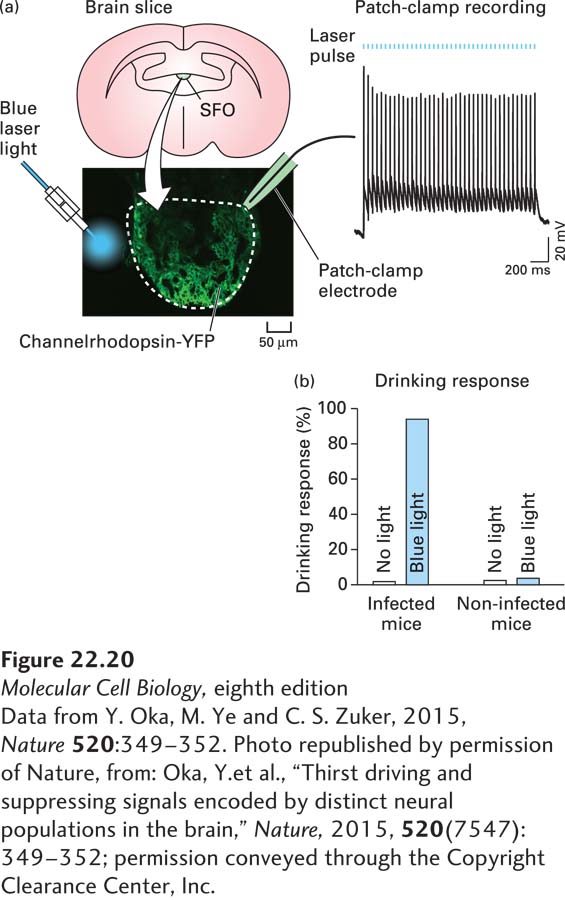
FIGURE 22-20 Using optogenetics to dissect neural circuits mediating thirst. (a) Channelrhodopsin tagged with a fluorescent protein (YFP) was expressed in excitatory neurons in the subfornical organ (SFO) of the hypothalamus using an excitatory neuron–specific promoter. Neurons expressing channelrhodopsin-YFP were confirmed to be excitable by illumination with blue light in acute hypothalamic slices. (b) When excitatory neurons in the SFO of living mice are activated by blue light, the mice seek water and drink large volumes even if they are well hydrated.
[Data from Y. Oka, M. Ye and C. S. Zuker, 2015, Nature 520:349–352. Photo republished by permission of Nature, from: Oka, Y. et al., “Thirst driving and suppressing signals encoded by distinct neural populations in the brain,” Nature, 2015, 520(7547):349–352; permission conveyed through the Copyright Clearance Center, Inc.]

