Neurotransmitters Are Transported into Synaptic Vesicles by H+-Linked Antiport Proteins
In this section, we focus on how neurotransmitters are packaged in membrane-bound synaptic vesicles in the axon terminus. Numerous small molecules function as neurotransmitters at various synapses. With the exception of acetylcholine, the most common neurotransmitters, as shown in Figure 22-25, are amino acids or derivatives of amino acids. Nucleotides such as ATP and the corresponding nucleosides, which lack phosphate groups, also function as neurotransmitters. While neurons were previously thought to make only one type of neurotransmitter, more recent studies indicate that some neurons can produce and release more than one neurotransmitter. While the types of neurotransmitters are varied and while they operate in different parts of the nervous system, all signaling by neurotransmitters results in one of two outcomes: either the induction of an electrical signal or its inhibition.
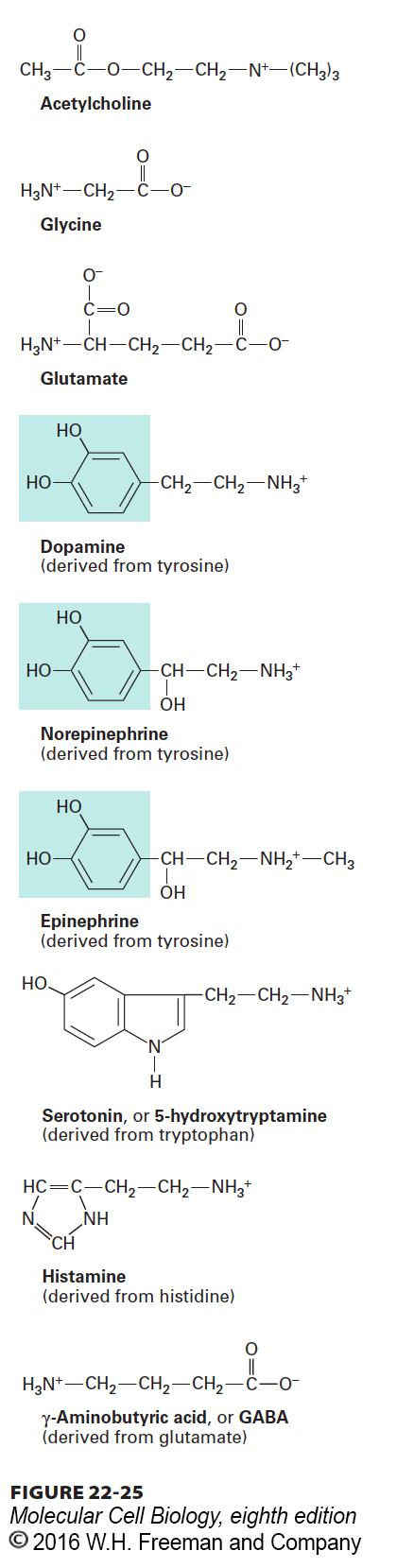
FIGURE 22-25 Structures of several small molecules that function as neurotransmitters. Except for acetylcholine, all of these molecules are amino acids (glycine and glutamate) or derived from the indicated amino acids. The three transmitters synthesized from tyrosine, which contain the catechol moiety (blue highlight), are referred to as catecholamines.
All of these neurotransmitters are synthesized in the cytosol and imported into membrane-bound synaptic vesicles within axon termini, where they are stored. These vesicles are 40–50 nm in diameter, and their lumen has a low pH, generated by operation of a V-class proton pump in the vesicle membrane. Similar to the accumulation of metabolites in plant vacuoles (see Figure 11-29), this proton concentration gradient (vesicle lumen > cytosol) powers neurotransmitter import by ligand-specific H+-linked neurotransmitter antiporters in the vesicle membrane (Figure 22-26).
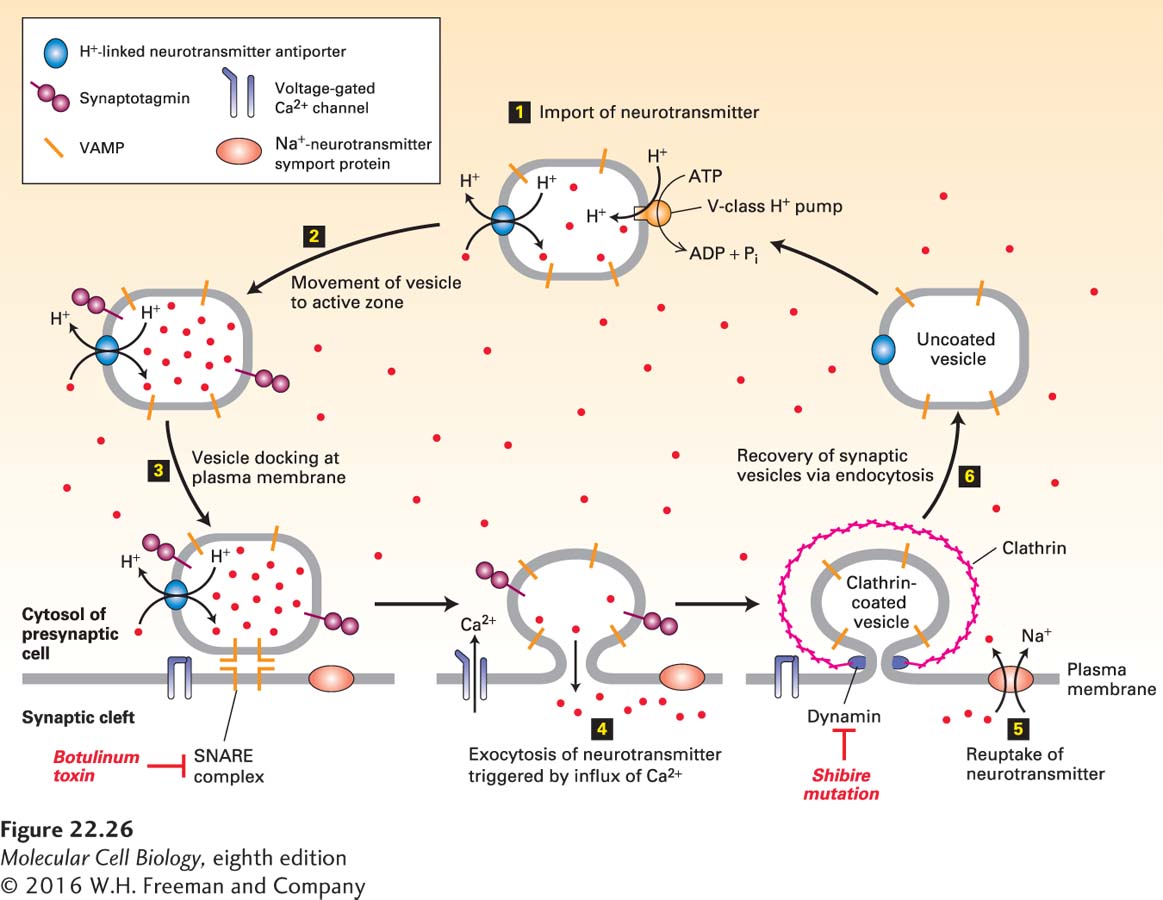
FIGURE 22-26 Cycling of neurotransmitters and of synaptic vesicles in axon termini. Most synaptic vesicles are formed by endocytic recycling as depicted here. The entire cycle typically takes about 60 seconds. Step 1: The uncoated vesicles express a V-type proton pump (orange) and a single type of H+-neurotransmitter antiporter (blue) specific for the particular neurotransmitter, to import neurotransmitters (red dots) from the cytosol. Step 2: Synaptic vesicles loaded with neurotransmitter move to the active zone. Step 3: Vesicles dock at defined sites on the plasma membrane of the presynaptic cell, and the vesicle v-SNAREs called VAMP bind to the plasma membrane t-SNAREs, forming a SNARE complex. Synaptotagmin prevents membrane fusion and release of neurotransmitter. Botulinum toxin prevents exocytosis by proteolytically cleaving VAMP, the v-SNARE on vesicles. Step 4: In response to a nerve impulse (action potential), voltage-gated Ca2+ channels in the plasma membrane open, allowing an influx of Ca2+ from the extracellular medium. The resulting Ca2+-induced conformational change in synaptotagmin leads to fusion of docked vesicles with the plasma membrane and release of neurotransmitters into the synaptic cleft. Synaptotagmin does not participate in the later steps of vesicle recycling or neurotransmitter import though it is still present. Step 5: Na+ symporter proteins take up neurotransmitter from the synaptic cleft into the cytosol, which limits the duration of the action potential and partially recharges the cell with transmitter. Step 6: Vesicles are recovered by endocytosis, creating uncoated vesicles, ready to be refilled and begin the cycle anew. After clathrin/AP vesicles containing v-SNARE and neurotransmitter transporter proteins bud inward and are pinched off in a dynamin-mediated process, they lose their coat proteins. Dynamin mutations such as shibire in Drosophila block the re-formation of synaptic vesicles, leading to paralysis. Unlike most neurotransmitters, acetylcholine is not recycled. See K. Takei et al., 1996, J. Cell Biol. 133:1237; V. Murthy and C. Stevens, 1998, Nature 392:497; and R. Jahn et al., 2003, Cell 112:519.
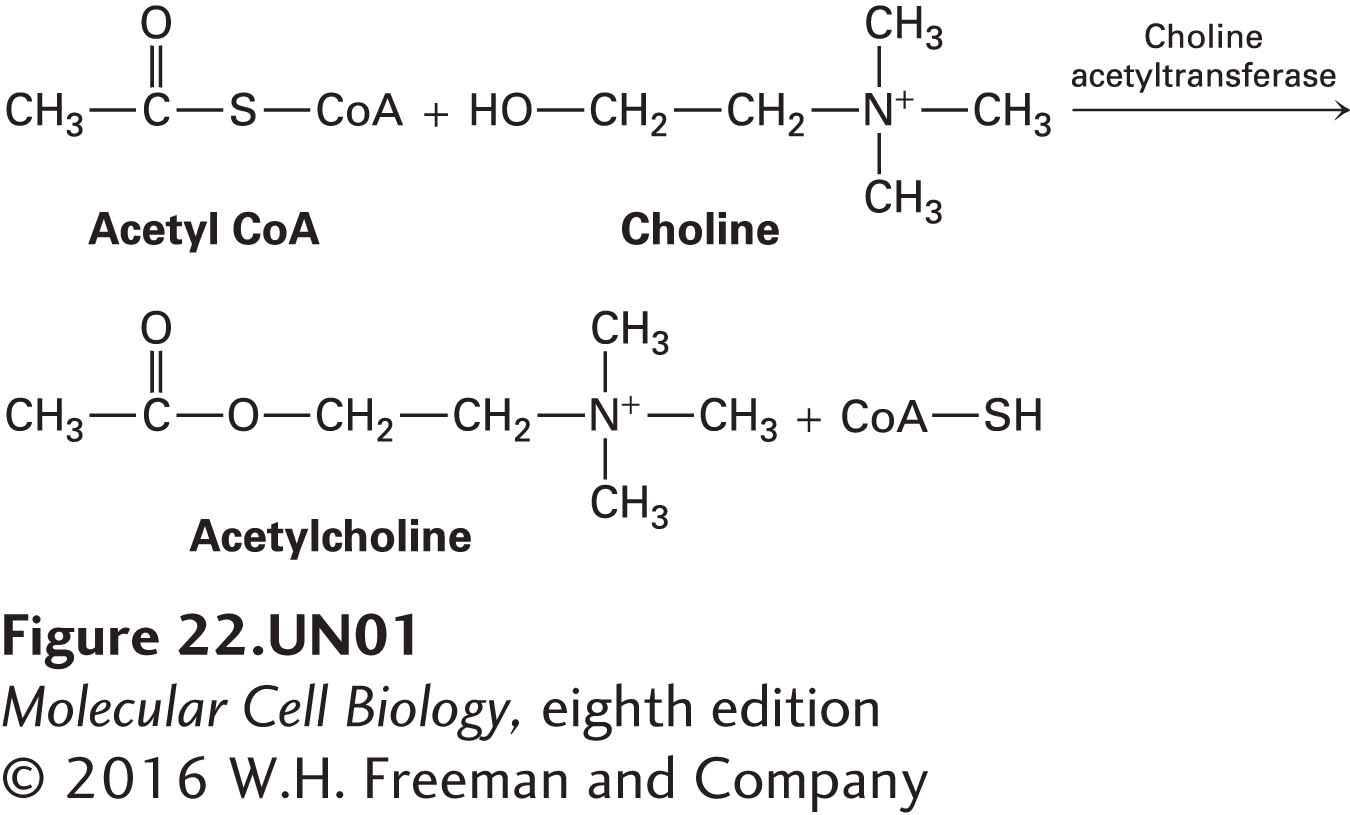
For example, acetylcholine is synthesized in the cytosol from acetyl coenzyme A (acetyl CoA), an intermediate in the degradation of glucose and fatty acids, and choline in a reaction catalyzed by choline acetyltransferase:
Synaptic vesicles take up and concentrate acetylcholine from the cytosol against a steep concentration gradient, using an H+/acetylcholine antiporter in the vesicle membrane. As with other antiporters, the export of protons from the forming vesicle down its electrochemical gradient powers the uptake of the neurotransmitter. As an example of an evolutionary mechanism to ensure coordinate expression of these two proteins, the gene encoding this antiporter is contained entirely within the first intron of the gene encoding choline acetyltransferase.
Different H+/neurotransmitter antiport proteins are used for import of other neurotransmitters into synaptic vesicles. For example, glutamate is imported into synaptic vesicles by a family of proteins called vesicular glutamate transporters (VGLUTs). VGLUTs are highly specific for glutamate but have rather low substrate affinity (Km = 1–3 mM). Another family of transporters, the vesicular GABA transporters (VGATs) transport GABA and glycine into synaptic vesicles. Like the acetylcholine transporter, VGLUTs and VGATs are antiporters, moving glutamate and GABA or glycine into synaptic vesicles while protons move in the other direction. VGLUT and VGAT serve as useful markers for excitatory and inhibitory synaptic terminals, respectively.