Mechanoreceptors Are Gated Cation Channels
Our skin, especially the skin of our fingers, is highly specialized for collecting sensory information. Our whole body, in fact, has numerous mechanosensors embedded in its various tissues. These sensors frequently make us aware of touch, the positions and movements of our limbs or head (proprioception), pain, and temperature, though we often go through periods when we ignore the inputs. Mammals use different sets of receptor cells to report on touch, temperature, and pain. These mechanosensory receptors are located at the terminals of a class of bipolar sensory neurons called dorsal root ganglion cells. The cell bodies of dorsal root ganglion cells are located in the dorsal root ganglion, adjacent to the spinal cord, and the neurons extend an axon that bifurcates into a peripheral branch that innervates the skin and contains the mechanosensory receptors, and a central branch that projects to the spinal cord or brain stem to relay sensory signals for processing.
1062
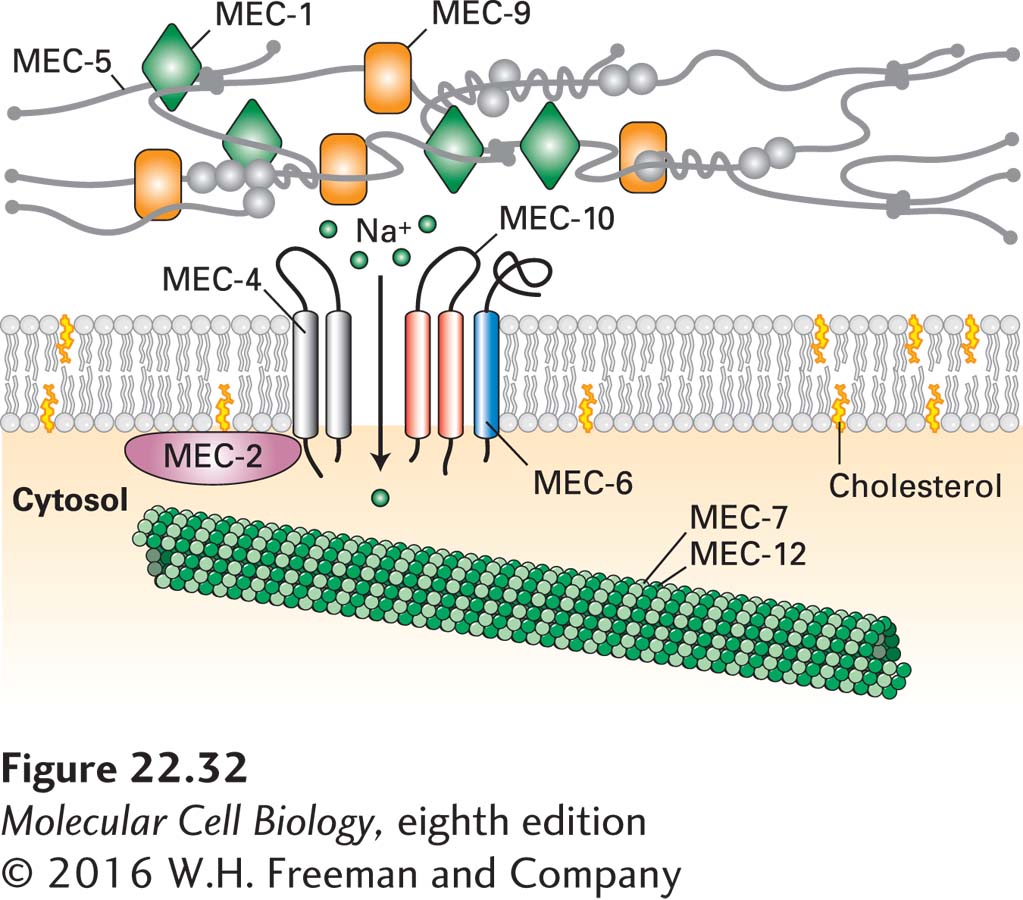
Many mechanosensory receptors are Na+ or Na+/Ca2+ channels that are gated, or opened, in response to specific stimuli; activation of such receptors causes an influx of Na+ or both Na+ and Ca2+ ions, leading to membrane depolarization. Examples include the stretch and touch receptors that are activated by stretching of the cell membrane; these have been identified in a wide array of cells, ranging from vertebrate muscle and epithelial cells to yeast, plants, and even bacteria.
The cloning of genes encoding touch receptors began with the isolation of mutant strains of Caenorhabditis elegans that were insensitive to gentle body touching. Three of the genes in which mutations were isolated—