Each Olfactory Receptor Neuron Expresses a Single Type of Odorant Receptor
1068
The key to understanding the specificity of the olfactory system is that in both mammals and insects each ORN produces only a single type of odorant receptor. Any electrical signal from that cell will convey to the brain a simple message: “an odorant is binding to my receptors.” Receptors are not always completely monospecific for odorants. Some receptors can bind more than one kind of molecule, but the molecules detected are usually closely related in structure. Conversely, some odorants bind to multiple receptors.
There are about 5 million ORNs in the mouse, so on average each of the 800 or so olfactory receptor genes is active in approximately 6000 cells. There are about 2000 glomeruli (roughly 2 for each odorant receptor gene), so on average the axons from a few thousand ORNs converge on each glomerulus (see Figure 22-38). From there about 25 mitral axons per glomerulus, or a total of 50,000 mitral neurons, connect to higher brain centers. Thus the initial odorant sensing information is carried directly to higher parts of the brain without processing, a simple report of what odorant has been detected.
The one neuron–
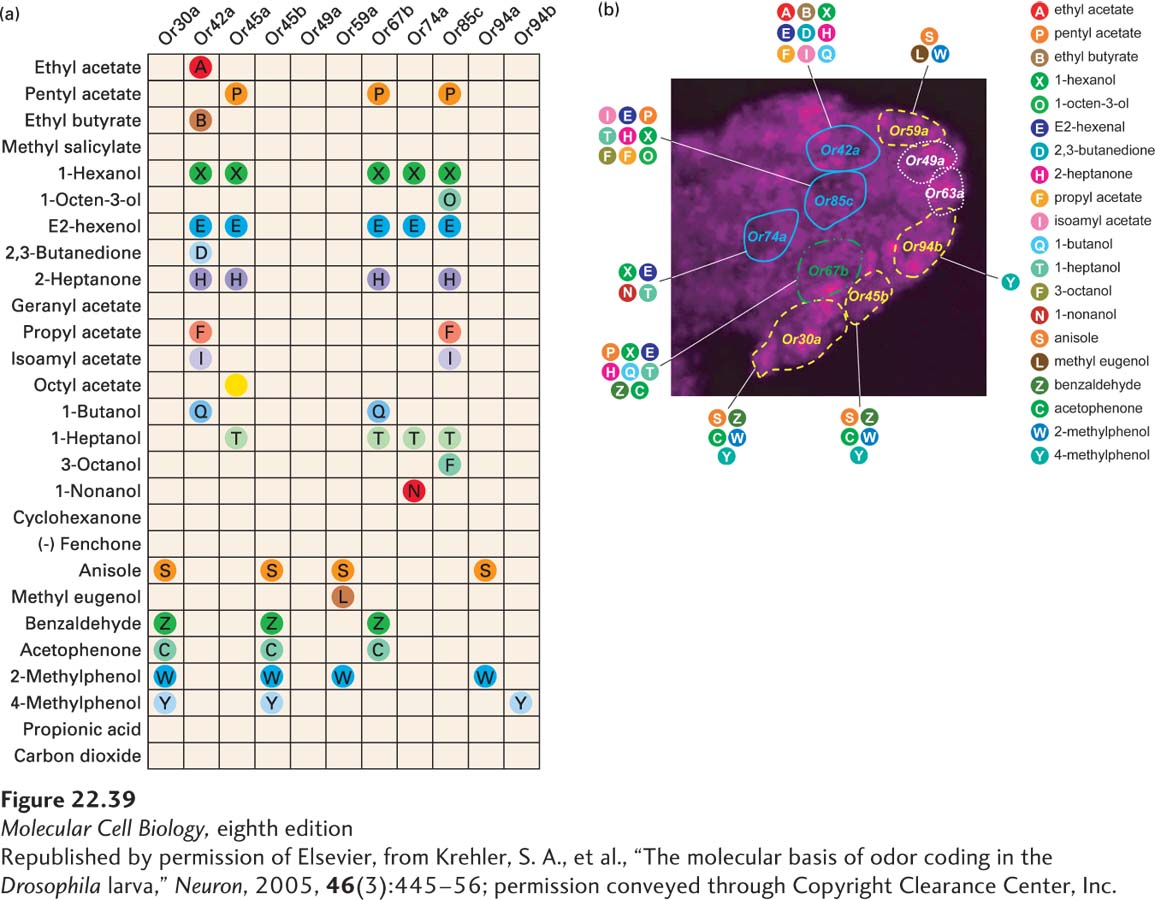
The simple system of having each cell make only one receptor type also has some impressive difficulties to overcome: (1) Each receptor must be able to distinguish a type of odorant molecule or a set of molecules with specificity adequate to the needs of the organism. A receptor stimulated too frequently would not be useful. (2) Each cell must express one and only one receptor gene product. All the other receptor genes must be turned off. At the same time, the collective efforts of all the cells in the nasal epithelium must allow the production of enough different receptors to give the animal adequate sensory versatility. It does little good to have genes for hundreds of receptors if most of them are never expressed, but it is a regulatory challenge to turn on one and only one gene in each cell and at the same time express all the receptor genes across the complete population of cells. (3) The neuronal wiring of the olfactory system must make discrimination among odorants possible so that the brain can determine which odorants are present. Otherwise the animal might be feeling at ease and relaxed when it should be running away as fast as possible.
1069
The solution to the first problem is the great variability of the olfactory receptor proteins, both within and between species. The solution to the second problem, the expression of a single olfactory receptor gene per cell, has been shown to involve a remarkable form of epigenetic silencing that assures that thousands of olfactory receptor alleles remain inactive in each ORN. These studies have shown that receptor choice relies on the selective activation of a single olfactory receptor gene from a developmental state in which all olfactory receptor genes are silenced. Activation is triggered by a histone demethylase and a specific adenylate cyclase, both of which are required to derepress the single olfactory receptor locus. The active and inactive genes are spatially segregated within the nucleus, with the inactive genes being buried in heterochromatic foci while the active genes are located in euchromatic domains (see Chapter 8).
The third problem, how the system is wired so the brain can understand which odor has been detected, has been partly answered. First, ORNs that express the same receptor send their axons to the same glomerulus. Thus all cells that respond to the same odorant send processes to the same destination. In mice, a crucial clue about the patterning of the olfactory system came from the discovery that olfactory receptors play two roles in ORNs: odorant binding and, during development, axon guidance. Multiple ORN axons expressing the same receptor are guided to the same glomerulus destination. Each olfactory receptor has a distinct, odorant-