During an Adaptive Response, B Cells Switch from Making Membrane-Bound Ig to Making Secreted Ig
As just described, the B-cell receptor, a membrane-bound IgM, provides a B cell with the ability to recognize a particular antigen, an event that triggers clonal selection and proliferation of that B cell, thus increasing the number of B cells specific for that antigen (see Figure 23-12). However, key functions of immunoglobulins, such as neutralization of antigens or killing of bacteria, require that those products be released by the B cell, so that they can accumulate in the extracellular environment and act at a distance from the site where they were produced.
Whether to synthesize membrane-bound or secreted immunoglobulin is a choice made by the B cell during processing of the heavy-chain primary transcript. As shown in Figure 23-19, the µ locus contains two exons (TM1 and TM2) that together encode a C-terminal domain that anchors IgM in the plasma membrane. One polyadenylation site is found upstream of these exons; a second polyadenylation site is present downstream. If the downstream poly(A) site is chosen, then further processing yields an mRNA that encodes the membrane-bound form of µ. (As described above, this choice is necessary for formation of the B-cell receptor, which includes membrane-bound IgM.) If the upstream poly(A) site is chosen, processing yields the secreted version of the µ chain. Similar arrangements are found for the other Ig constant-region gene segments (γ, α, ε), each of which can specify either a membrane-bound or a secreted heavy chain. The ability to switch between the membrane-anchored and the secreted form of immunoglobulin heavy chains by alternative use of polyadenylation sites (not by alternative splicing) is so far unique to this family of gene products.
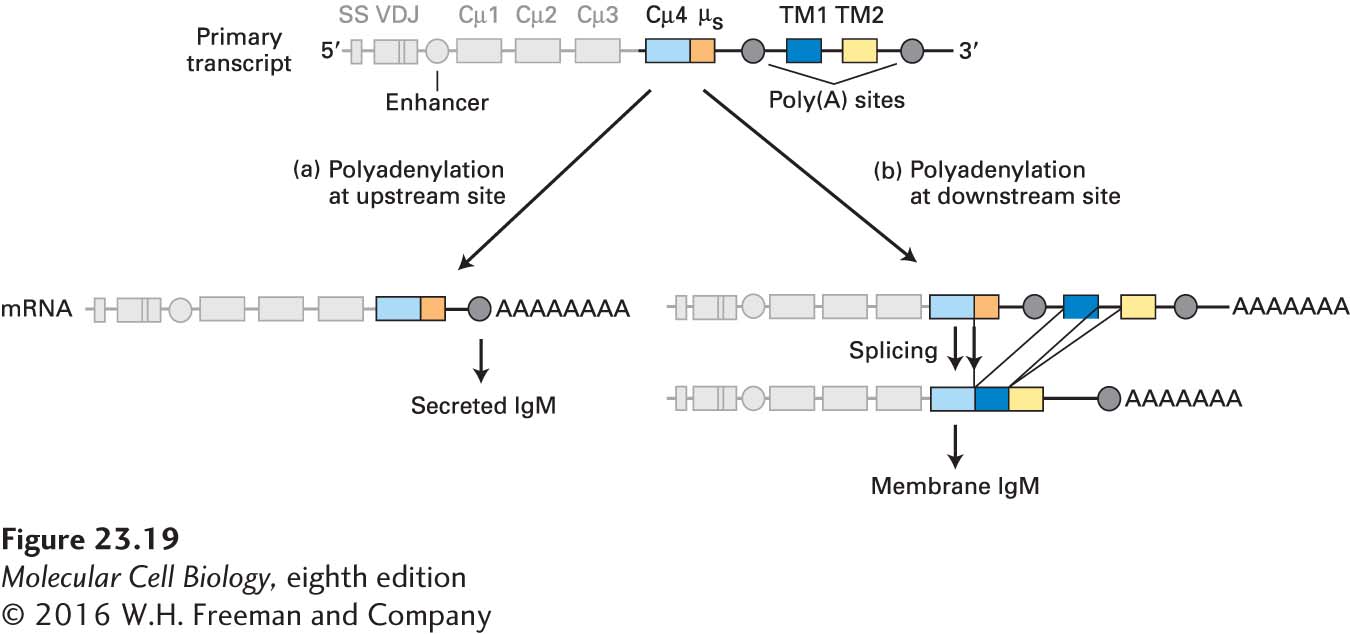
FIGURE 23-19 Synthesis of secreted and membrane IgM. The organization of the µ heavy-chain primary transcript is shown at the top: Cµ4 is the exon encoding the fourth µ constant-region domain; µs is a coding sequence unique for secreted IgM; TM1 and TM2 are exons that specify the transmembrane domain of the µ chain. Whether secreted or membrane-bound IgM is made depends on which poly(A) site is selected during processing of the primary transcript. (a) If the upstream poly(A) site is used, the resulting mRNA includes the entire Cµ4 exon and specifies the secreted form of the µ chain. (b) If the downstream poly(A) site is used, a splice donor site in the Cµ4 exon allows splicing to the transmembrane exons, yielding a mRNA that encodes the membrane-bound form of the µ chain. Similar mechanisms generate secreted and membrane-bound forms of other Ig isotypes. SS = signal sequence.
The capacity to switch from the synthesis of exclusively membrane-bound immunoglobulin to the synthesis of secreted immunoglobulin is acquired by B cells in the course of their differentiation. Terminally differentiated B cells, called plasma cells, are devoted almost exclusively to the synthesis of secreted antibodies (see Figure 23-7). Each plasma cell synthesizes and secretes several thousand antibody molecules per second. It is this ramped-up production of secreted antibodies that underlies the effectiveness of the adaptive immune response in eliminating a pathogen and protecting against subsequent infection with the same pathogen. The protective value of antibodies is proportional to the concentration at which they are present in the circulation. Indeed, circulating antibody levels are often used as the key parameter to determine whether vaccination against a particular pathogen has been successful. The ability of plasma cells to secrete large amounts of immunoglobulins requires a massive expansion of the endoplasmic reticulum, a hallmark of plasma cells. The unfolded-protein response (see Chapter 13) is initiated in B cells as an essential physiological mechanism to expand the ER and prepare the differentiating B cell for its future task as a highly active secretory cell. Interference with the unfolded-protein response abolishes the ability of B cells to turn into plasma cells.
