MHC Molecules Bind Peptide Antigens and Interact with the T-Cell Receptor
Both class I and class II MHC molecules are highly polymorphic; that is, many allelic variants exist among individuals of the same species. The vertebrate immune system can respond to these allelic differences, and its ability to recognize allelic MHC variants is the underlying immunological cause for rejection of transplants that involve unrelated, genetically distinct individuals. Yet the two classes of MHC molecules are also structurally similar in many respects, as are their interactions with peptides and the T-cell receptor (Figure 23-23).
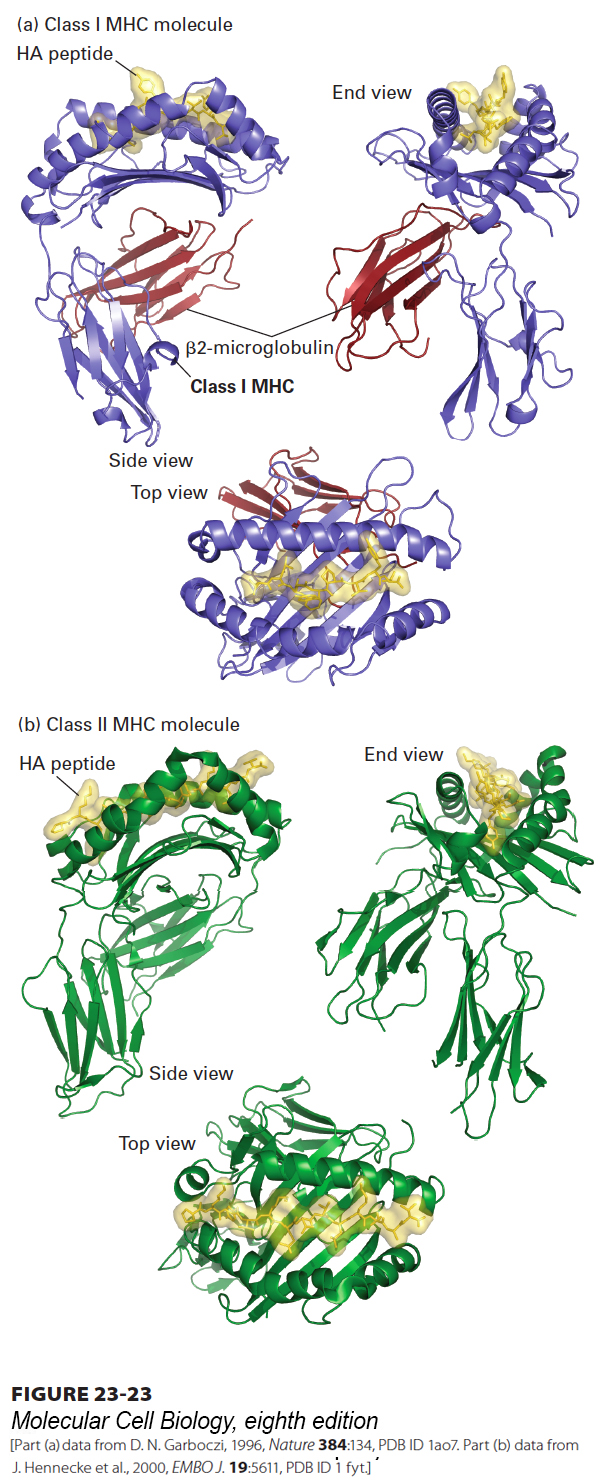
FIGURE 23-23 Three-dimensional structure of class I and class II MHC molecules. (a) Shown here is the structure of a class I MHC molecule with bound antigenic (HA) peptide as determined by x-ray crystallography. The portion of a class I MHC molecule that binds a peptide consists of a β sheet composed of eight β strands and flanked by two α helices. The peptide-binding cleft is formed entirely from the MHC-encoded large subunit, which associates noncovalently with the small subunit (β2-microglobulin) encoded elsewhere. (b) Class II MHC molecules are structurally similar to class I molecules, but with several important distinctions. Both the α and β subunits of class II MHC molecules are MHC encoded and contribute to formation of the peptide-binding cleft. The peptide-binding cleft of class II MHC molecules accommodates a wider range of peptide sizes than that of class I molecules. The extracellular portions of class I and class II MHC products, both of which are type I membrane proteins, contain a transmembrane segment and a cytoplasmic tail (see Figures 23-21, 23-26, and 23-29), not included in the crystallographic analysis.
[Part (a) data from D. N. Garboczi, 1996, Nature 384:134, PDB ID 1ao7. Part (b) data from J. Hennecke et al., 2000, EMBO J. 19:5611, PDB ID 1 fyt.]
There are many polymorphisms (genetic differences comprising multiple allelic variants at a given locus) in the genes that encode class I and class II MHC molecules. There are more than 2000 distinct alleles for all human MHC products combined. MHC molecules are particularly important for recognizing “self” tissue and distinguishing it from “nonself” (and thus possibly pathogenic) substances. In general, except for close relatives, any two individuals have a very low chance of sharing the same MHC variants. Any inter-individual differences in MHC molecules in a graft recipient and donor will be recognized by the recipient’s immune system, which will treat the graft as foreign and eliminate it (graft rejection). The greater the similarity in the set of MHC alleles of a donor and a transplant recipient, the greater the chance that the transplant will be accepted. This is why surgeons look for an MHC “matched” individual to donate an organ. If the tissue type (MHC alleles) of the donor does not exactly match that of the recipient, it is necessary to use drugs that suppress the immune responses of the recipient to prevent organ rejection.
The cell-biological mechanisms by which the immune system develops the capacity to distinguish “self” from “nonself” (or pathogenic from nonpathogenic) are complex, yet worth understanding. Understanding the molecular and cellular basis of immunity has enormous practical consequences for medicine and public health. We will therefore consider these molecular and cellular mechanisms in detail.
Class I MHC Molecules Class I MHC molecules, which belong to the Ig superfamily, consist of two polypeptide subunits. The larger subunit, for which there are multiple independent gene copies in the MHC region of mammalian genomes, is a type I membrane glycoprotein (see Figure 13-10). The smaller β2-microglobulin subunit is not encoded by the MHC and corresponds in structure to an Ig domain. The larger subunits of class I MHC molecules in humans are encoded by the HLA-A, HLA-B, and HLA-C loci (see Figure 23-20), each of which displays extensive allelic variation among individuals. In the mouse, the larger subunits of class I MHC molecules are encoded by the H-2K and H-2D loci, each likewise with many known allelic variants.
The three-dimensional structure of a class I MHC molecule reveals two membrane-proximal Ig-like domains (see Figure 23-23a). These domains support an eight-stranded β sheet topped by two α helices. Jointly, the β sheet and the helices create a cleft, closed at both ends, in which a peptide binds. The mode of peptide binding by a class I MHC molecule requires that the peptide be about 8–10 amino acids long, so that the ends of the peptide can be tucked into pockets that accommodate the charged amino and carboxyl groups at the termini. Further, the peptide is anchored into the peptide-binding cleft by means of a small number of amino acid side chains, each of which is accommodated by a pocket in the MHC molecule that neatly fits that particular amino acid residue (Figure 23-24a). On average, two such “specificity pockets” must be filled correctly to allow stable peptide binding, restricting binding to peptides with side chains that can fit into these pockets. In this manner, a given MHC molecule can accommodate a large number of peptides of diverse, yet circumscribed, sequence.
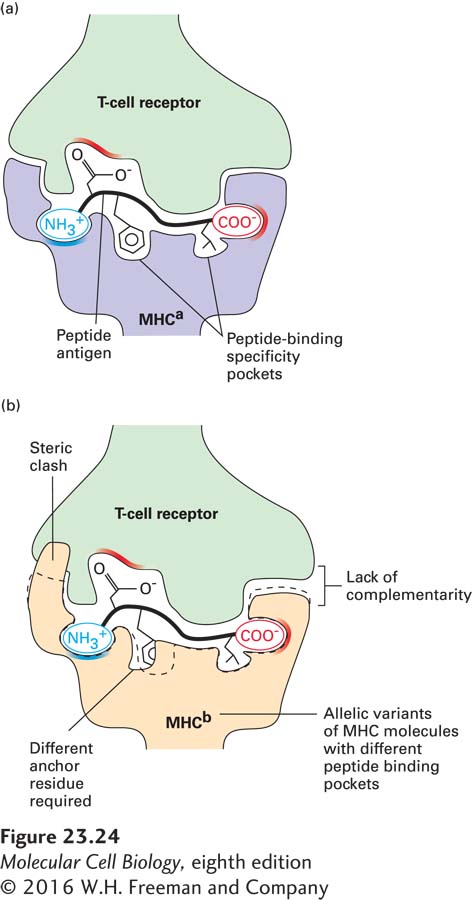
FIGURE 23-24 Peptide binding and MHC restriction. (a) Peptides that bind to class I molecules are on average 8–10 residues in length, require proper accommodation of the termini, and include two or three residues that are conserved (anchor residues). Positions in class I molecules that distinguish one allele from another (polymorphic residues) occur in and around the peptide-binding cleft. The polymorphic residues in the MHC affect both the specificity of peptide binding and interactions with T-cell receptors. Successful “recognition” of an antigenic peptide–MHC complex by a T-cell receptor requires a good fit among the receptor, peptide, and MHC molecule. (b) Steric clash and a lack of complementarity between anchor residues and the MHC molecule prevent proper binding. T-cell receptors are thus restricted to binding specific peptide-MHC complexes.
The polymorphic residues that distinguish one allelic MHC molecule from another are located mainly in and around the peptide-binding cleft. These residues therefore determine the architecture of the peptide-binding pocket and hence the specificity of peptide binding. Further, these polymorphic residues affect the surface of the MHC molecule that makes contact with the T-cell receptor. A T-cell receptor that can interact with one particular class I MHC allele will therefore, as a rule, not interact with unrelated MHC molecules because of their different surface architectures (Figure 23-24b); this is the molecular basis of MHC restriction. The CD8 molecule on cytotoxic T cells functions as a co-receptor, binding to conserved portions of the class I MHC molecule. The presence of CD8 thus “sets” the class I MHC preference of any mature T cell that bears it.
Class II MHC Molecules The two subunits (α and β) of class II MHC molecules are both type I membrane glycoproteins of the Ig superfamily. The typical mammalian MHC contains several loci that encode class II MHC molecules (see Figure 23-21). Like the large subunit of class I molecules, both the α and β subunits of class II molecules show genetic polymorphism.
The basic three-dimensional design of class II MHC molecules resembles that of class I MHC molecules: two membrane-proximal Ig-like domains support a peptide-binding portion with a peptide-binding cleft (see Figure 23-23b). In class II MHC molecules, the α and β subunits contribute equally to the construction of the peptide-binding cleft. This cleft is open at both ends and thus supports the binding of peptides longer than those that bind to class I MHC molecules because the peptides can protrude from both ends of the cleft. The mode of peptide binding involves pockets that accommodate specific peptide side chains as well as contacts between side chains of the MHC molecule and main-chain atoms of the bound peptide. As for class I MHC, class II MHC polymorphisms mainly affect residues in and around the peptide-binding cleft, so that peptide-binding specificity usually differs among different allelic products. A T-cell receptor that interacts with a particular class II MHC molecule will not, as a rule, interact with a different class II MHC allelic variant, not only because of the difference in the peptide-binding specificity of the allelic MHC molecules, but also because of the polymorphisms that affect the residues that contact the T-cell receptor; as for class I MHC, this is the basis for class II MHC restricted recognition of antigens.
As we will see below, class II MHC molecules evolved to present peptides generated predominantly in endosomes and lysosomes. Binding of peptides to a class II MHC molecule takes place in those organelles, and class II MHC molecules are targeted specifically to those locations after their synthesis in the endoplasmic reticulum. This targeting is accomplished by means of a chaperone called the invariant chain, a type II membrane glycoprotein (see Figure 13-10). The invariant chain (Ii) plays a key role in the early stages of class II MHC biosynthesis by forming a trimeric structure onto which three class II MHC αβ heterodimers assemble. The final assembly product thus consists of nine polypeptides: (αβIi)3. The interaction between Ii and the αβ heterodimer involves a stretch of Ii called the CLIP segment, which occupies the class II MHC peptide-binding cleft. Once the (αβIi)3 complex is assembled, the complex enters the secretory pathway and is diverted to endosomes and lysosomes at the trans-Golgi network (see Figure 14-1). The signals responsible for this diversion are carried by the Ii cytoplasmic tail and do not obviously conform to the pattern of endosomal targeting or retrieval signals commonly found on lysosomal membrane proteins. Some of the (αβIi)3 complexes are directed straight to the cell surface, from which they may be internalized, but the vast majority end up in late endosomes.
As we saw for class I MHC molecules and their CD8 co-receptor, the CD4 co-receptor recognizes conserved features on class II MHC molecules. Any mature T cell that bears the CD4 co-receptor uses class II MHC molecules for antigen recognition.

