Signaling via Antigen-Specific Receptors Triggers Proliferation and Differentiation of T and B Cells
The immune responses mediated by T cells and B cells are initiated when their antigen-specific cell-surface receptors (TCRs or BCRs) are activated by binding to their respective ligands. The ligands for TCRs are MHC-peptide complexes expressed on the surfaces of APCs. The ligands for BCRs are antigens that bind to the receptors without the need for MHC intervention and do not need to be associated with a presenting cell. The activation of TCRs and BCRs by their antigens is similar to the activation of the signaling receptors we have already considered (G protein–coupled receptors, tyrosine kinase receptors; see Chapters 15 and 16) in that signal transduction cascades are activated. Several integral membrane proteins, as well as soluble cytosolic proteins, participate in TCR and BCR signaling. In some cases, these membrane-associated proteins can be thought of as auxiliary subunits of the receptors. Examples of how such auxiliary proteins participate in signaling are shown in Figure 23-31. The cytosolic portions of the antigen-specific receptors themselves are very short, do not protrude much beyond the cytosolic leaflet of the plasma membrane, and are incapable of recruitment of downstream signaling molecules. Instead, as discussed previously, the antigen-specific receptors associate with auxiliary subunits that contain ITAMs. Engagement of the antigen-specific receptors by ligand initiates a series of receptor-proximal events: kinase activation, modification of ITAMs, and subsequent recruitment of adapter proteins that serve as scaffolds for recruitment of yet other downstream signaling molecules.
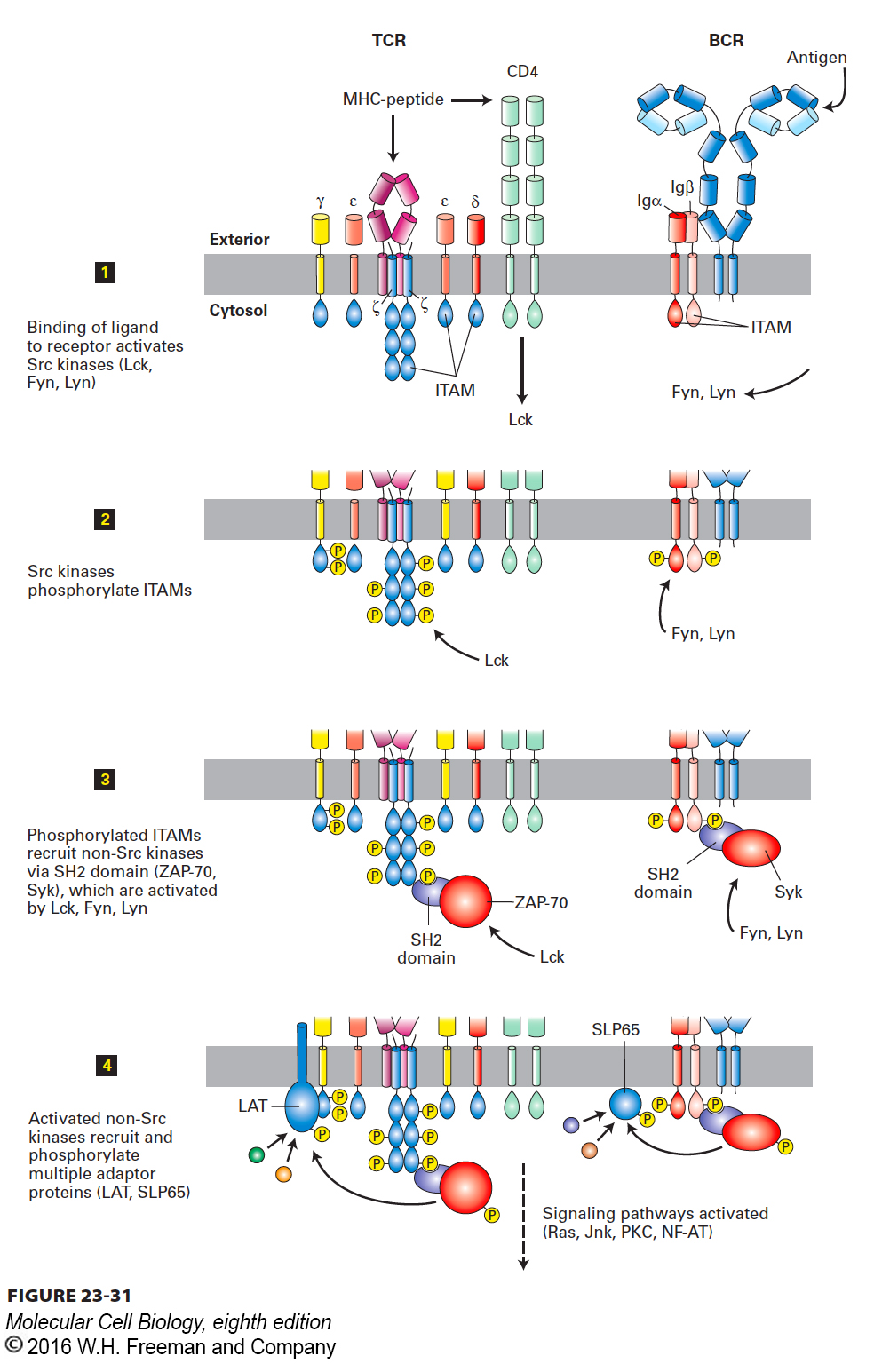
FIGURE 23-31 Signal transduction from the T-cell receptor (TCR) and B-cell receptor (BCR). The signal transduction pathways used by the antigen-specific receptors of T cells (left) and B cells (right) are conceptually similar. The initial stages are depicted in this figure; downstream signaling events lead to changes in gene expression that result in proliferation and differentiation of the antigen-stimulated lymphocytes. See text for further discussion.
As outlined in Figure 23-31, engagement of the antigen-specific receptors by ligand activates Src-family tyrosine kinases (e.g., Lck in helper T cells; Lyn and Fyn in B cells). These kinases are found in close proximity to or physically associated with the antigen-specific receptors. The active kinases phosphorylate the ITAMs in the antigen-specific receptors’ auxiliary subunits. In their phosphorylated forms, these ITAMs recruit and activate non-Src-family tyrosine kinases (ZAP-70 in T cells, Syk in B cells) as well as other adapter proteins. This recruitment and activation involves phosphoinositide-specific phospholipase Cγ and PI-3 kinases. Subsequent downstream events parallel those described in Chapter 16 for signaling from receptor tyrosine kinases. Antigen-specific receptors on B and T cells are perhaps best characterized as “modular” receptor tyrosine kinases, with the ligand recognition units and kinase domains carried by separate molecules. Ultimately, signaling via antigen-specific receptors initiates transcription programs that determine the fate of the activated lymphocyte: proliferation and differentiation.
T cells depend critically on the cytokine interleukin 2 (IL-2) for clonal expansion. Following antigen stimulation of a T cell, one of the first genes to be turned on is that for IL-2. The T cell responds to its own initial burst of IL-2 and proceeds to make more IL-2, an example of autocrine stimulation and part of a positive feedback loop. An important transcription factor required for the induction of IL-2 synthesis is the NF-AT protein (nuclear factor of activated T cells). This protein is sequestered in the cytoplasm in phosphorylated form and cannot enter the nucleus unless it is dephosphorylated first. The phosphatase responsible is calcineurin, a Ca2+-activated enzyme. The initial rise in cytosolic Ca2+ leading to activation of calcineurin results from mobilization of ER-resident Ca2+ stores triggered by hydrolysis of PI(4,5)P2 and the concomitant generation of IP3 (see Figure 15-34, steps 2–4).
The immunosuppressant drug cyclosporine inhibits calcineurin activity through formation of a cyclosporine-cyclophilin complex, which binds and inhibits calcineurin. If dephosphorylation of NF-AT is suppressed, NF-AT cannot enter the nucleus and induce transcription of the IL-2 gene. This precludes clonal expansion of antigen-stimulated T cells and so leads to immunosuppression, arguably the single most important intervention that contributes to the success of organ transplantation involving unrelated donors and recipients (individuals who are genetically different and therefore express different MHC products), referred to as allogeneic tissue transplantation. Although the success of transplantation varies with the organ used, the availability of strong immunosuppressants such as cyclosporine has expanded the possibilities of clinical transplantation enormously.
