T Cells Capable of Recognizing MHC Molecules Develop Through a Process of Positive and Negative Selection
The rearrangement of the gene segments that are assembled to encode a functional T-cell receptor is a stochastic event, completed on the part of the T cell without any prior knowledge of the MHC molecules with which its receptors must ultimately interact. As in somatic recombination of Ig heavy-chain loci in B cells, the first gene segments to be rearranged in the TCR β chain are the D and J elements; a V segment is then joined to the newly recombined DJ (see Figure 23-30). At this stage of T-cell development, productive rearrangement allows the synthesis of the TCR β chain, which is incorporated into the pre-TCR through association with the pre-T α subunit. This pre-TCR fulfills a function strictly analogous to that of the pre-BCR in B-cell development: it tells the T cell that it has successfully completed a productive rearrangement, with no need for further rearrangements in the genes on the homologous chromosome. The pre-TCR allows clonal expansion of the pre-T cells that successfully underwent rearrangement, and it imposes allelic exclusion to ensure that, as a rule, a single functional TCR β subunit is generated for a given T cell and its descendants. RAG expression subsides until the expansion phase of the pre-T cells is complete, after which it is re-initiated to allow rearrangement of the TCR α locus, ultimately leading to the generation of T cells with a fully assembled TCR. Figure 23-32 illustrates the analogous steps in the development of T and B cells.
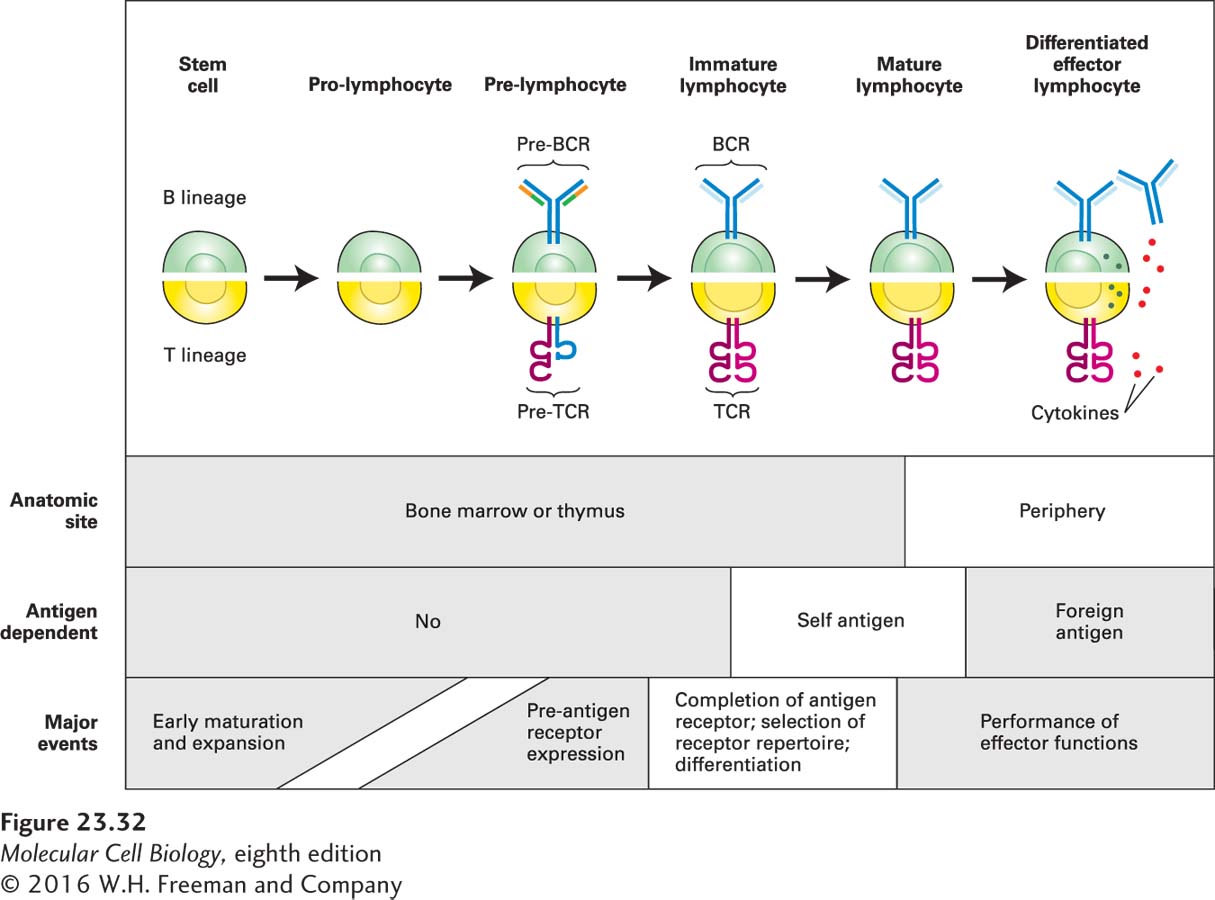
FIGURE 23-32 Comparison of T-cell and B-cell development. Cell fate decisions are executed by receptors composed of either the newly rearranged µ chain (pre-BCR) or the newly rearranged β chain (pre-TCR). The pre-BCRs and pre-TCRs serve similar functions: signaling clonal expansion of cells that have successfully undergone rearrangement and allelic exclusion. This phase of lymphocyte development does not require antigen recognition. Both the pre-BCR and pre-TCR include subunits unique to each receptor type and absent from the antigen-specific receptors found on mature lymphocytes: VpreB and λ5 (orange, green) for the pre-BCR; pre-T α (blue) for the pre-TCR. Upon completion of the expansion phase, expression of the gene encoding the remaining subunit of the antigen-specific receptor begins: Ig light chain (light blue) for the BCR; TCR α chain (light red) for the TCR. Lymphocyte development and differentiation occur at distinct anatomic sites, and only fully assembled antigen-specific receptors (BCR, TCR) recognize antigen. Mature lymphocytes are strictly dependent on antigen recognition for their activation.
How is the newly emerging repertoire of T cells, with their diverse pre-TCRs, further differentiated so that a productive interaction with self-MHC–peptide complexes can occur? The random nature of the gene rearrangement process and the enormous variability engendered as a consequence produces a large and diverse set of TCRs, the vast majority of which cannot interact productively with the host MHC products, and are therefore useless. The immune system has developed selection processes to eliminate those T cells that make TCRs incapable of a productive interaction with self-MHC–peptide complexes. Selection in the thymus also removes those T cells with TCRs that can strongly interact with self-MHC–self-peptide complexes because such T cells have the potential to be self-reactive, damaging normal healthy tissue (autoimmunity). T cells whose TCRs recognize peptide-MHC complexes in the thymus with an affinity that falls between these two extremes receive survival signals and are positively selected. Recall that antigen processing and presentation are constitutive processes, so that in the thymus, all self-MHC molecules are necessarily occupied with peptides derived from self-proteins. These combinations of self-peptides complexed to class I and class II MHC molecules constitute the substrate used by the set of newly generated T-cell receptors to determine what is “self” and ought to be ignored.
The heterogeneity of peptide-MHC complexes presented to T cells undergoing selection makes it highly probable that the T-cell receptor interprets signals not only in a qualitative (strength, duration) manner, but also in additive fashion: the summation of the binding energies of the different MHC–self-peptide combinations it encounters helps determine the outcome of selection. This phenomenon is called the avidity model of T-cell selection.
T cells are killed off by apoptosis only if the appropriate self antigen is represented adequately in the thymus in the form of MHC-peptide complexes. How does the immune system ensure that T cells generated in the thymus learn to ignore self antigens that are not normally expressed at that location? Proteins that are expressed in tissue-specific fashion or after the development of the thymus, such as insulin in the β cells of the pancreas or the components of the myelin sheath in the nervous system, obviously fit this category. However, a factor called AIRE (autoimmune regulator) allows expression of such tissue-specific antigens in a subset of epithelial cells in the thymus. How AIRE accomplishes this is not known, but it is widely suspected of directly regulating the transcription of the relevant genes in the thymus and at select sites in secondary lymphoid organs. Defects in AIRE lead to a failure to express these tissue-specific antigens in the thymus. In individuals who do not express AIRE, developing T cells fail to receive the full set of instructions in the thymus that lead to the elimination of potentially self-reactive T cells. As a consequence, these individuals show a bewildering array of autoimmune responses, causing widespread tissue damage and disease.
