Innate Immunity Provides a Second Line of Defense
The innate immune system is activated once the mechanical and chemical defenses have failed and the presence of an invader is sensed (see Figure 23-1). The innate immune system comprises cells and molecules that are immediately available for responding to pathogens. Phagocytes, cells that ingest and destroy pathogens (see Figure 17-19), are widespread throughout tissues and epithelia and can be recruited to sites of infection. Several soluble proteins that are constitutively present in the blood or produced in response to infection or inflammation also contribute to innate defenses. Animals that lack an adaptive immune system, such as insects, rely exclusively on innate defenses to combat infections. Likewise, plants rely exclusively on innate defenses and lack adaptive immunity altogether.
Phagocytes and Antigen-Presenting Cells The innate immune system includes macrophages, neutrophils, and dendritic cells. All of these cells are phagocytic and come equipped with pathogen recognition receptors such as Toll-like receptors (TLRs); see Figure 23-35 for their molecular structure) and scavenger receptors on their cell surface. These receptors detect broad patterns of pathogen-specific markers, such as bacterial cell-wall constituents or nucleic acids that contain unmethylated CpG or double-stranded RNA, and are thus key sensors for detecting the presence of bacteria or viruses. When these markers bind to TLRs, the cells produce effector molecules, including antimicrobial peptides. Dendritic cells and macrophages whose TLRs have detected pathogens also function as antigen-presenting cells (APCs) by processing and displaying foreign materials to antigen-specific T cells, thus bridging the innate and adaptive immune systems. The structure and function of TLRs and their role in activating dendritic cells are described in detail in Section 23.6.
Inflammasomes and Non-TLR Nucleic Acid Sensors Mammalian cells possess a family of proteins, endowed with leucine-rich repeats, that are capable of recognizing all manner of nonself components and of perceiving “danger” signals. The molecules recognized by these proteins span a range from components of the bacterial cell wall to uric acid crystals, to heme degradation products, and even to asbestos and silica (Figure 23-4). Once recognized, these “danger” signals activate the assembly of a multiprotein complex called the inflammasome, which activates the effector proteins involved in inflammation. Proteins that make up the inflammasome contain modules that mediate interactions with adapter proteins that ultimately allow a physical connection with and activation of caspase-1, an enzyme that is critical in the production of cytokines that cause inflammation (a process described below). As we will see in Section 23.6, the inflammasome plays an important role in bridging the innate and adaptive immune response.
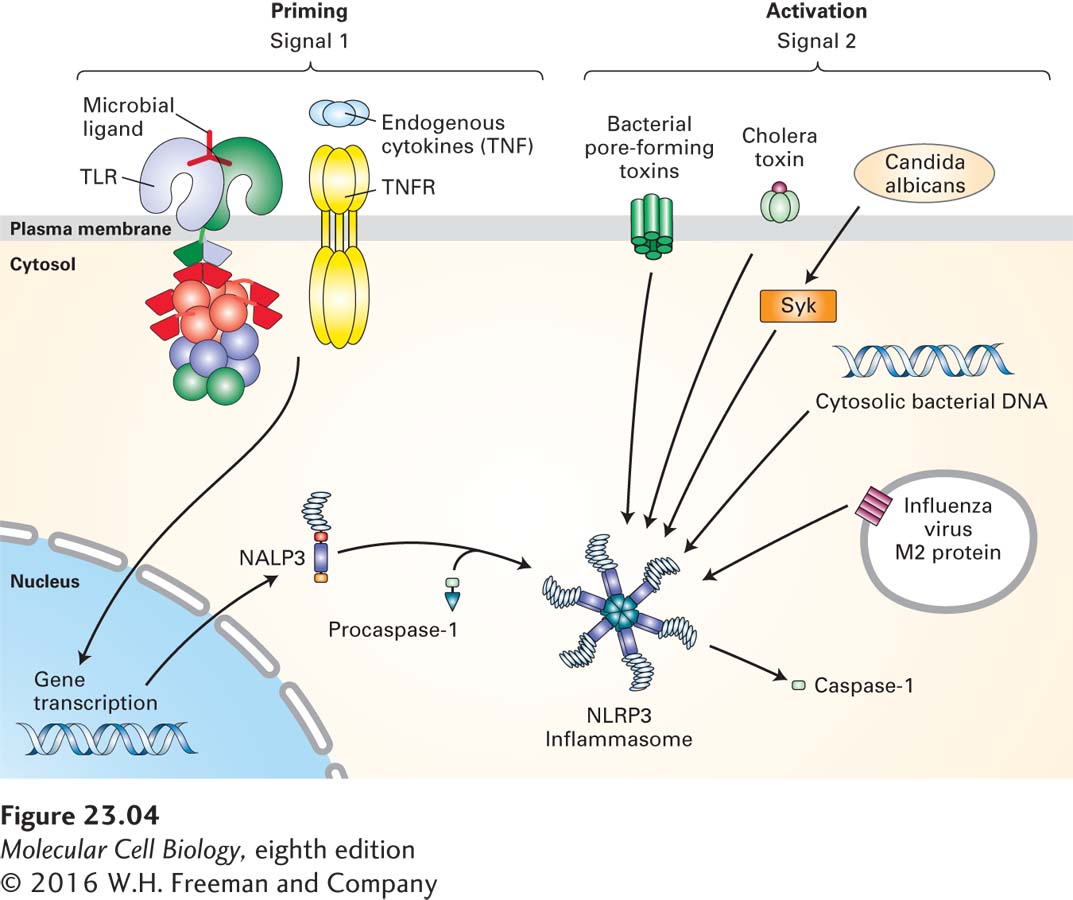
FIGURE 23-4 The NLRP3 inflammasome. The NLRP3 inflammasome activates caspase-1 only after receiving two signals. Signal 1 is provided by microbial antigens recognized via Toll-like receptors (TLRs) or by binding of endogenous cytokines such as TNF to the TNF receptor (TNFR). Signal 1 causes the up-regulation of NLRP3 and pro-IL-1β. Signal 2, which activates the NLRP3 inflammasome, can be provided by bacterial pore-forming toxins, by influenza virus M2 protein, by fungal particles via the kinase Syk (as shown for Candida albicans), or by cholera toxin (CT). Cytosolic bacterial DNA can also activate the NLRP3 inflammasome, although the molecular details of this mechanism are not yet understood.
Some mammalian TLRs that can recognize bacterial or viral nucleic acids have their ligand-binding domains in the lumen of endosomes. Mammalian cells also possess other sensors capable of detecting the presence of cytosolic nucleic acids. RIG-I and MDA5 are proteins specialized in recognition of viral RNA. Mammalian cells also possess an enzyme, cGAS, that is capable of generating cyclic dinucleotides from bacterial or viral DNA. These cyclic dinucleotides are then recognized by the ER-localized STING protein. Activation of these classes of receptors triggers inflammation and helps initiate an adaptive immune response.
The Complement System Another important component of the innate immune system is the complement system, a collection of serum proteins that can bind directly to microbial or fungal surfaces. This binding activates a proteolytic cascade that culminates in, among other things, the formation of a membrane attack complex, which is capable of forming pores in the pathogen’s protective membrane (Figure 23-5). The cascade of complement activation is conceptually similar to the blood-clotting cascade, with amplification of the reaction at each successive stage of activation. At least three distinct pathways can activate the complement system. The classical pathway requires the presence of antibodies produced in the course of an adaptive immune response and bound to their antigens on the surface of the target microbe. How such antibodies are produced will be described below. This complement pathway represents an example of components of the innate immune system acting together with the antibodies produced by adaptive immune system.
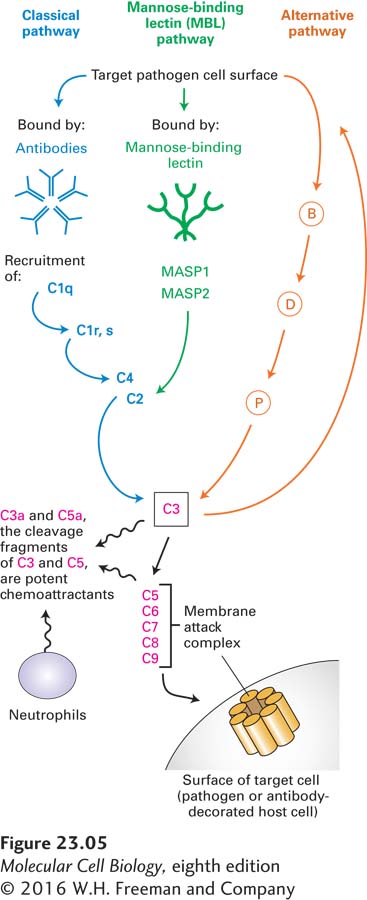
FIGURE 23-5 Three pathways of complement activation. The classical pathway involves the formation of antibody-antigen complexes. In the mannose-binding lectin pathway, mannose-rich structures found on the surfaces of many pathogens are recognized by mannose-binding lectin. The alternative pathway requires deposition of a special form of the serum protein C3, a major complement component, onto a microbial surface, upstream of which are factors B, D and P. Each of the activation pathways is organized as a cascade of proteases in which the downstream component is itself a protease. Amplification of activity occurs with each successive step. All three pathways converge on C3, which cleaves C5 and thus triggers formation of the membrane attack complex, leading to destruction of target cells. The small fragments of C3 and C5 generated in the course of complement activation initiate inflammation by attracting neutrophils, phagocytic cells that can kill bacteria at short range or upon ingestion.
In addition to the classical pathway of complement activation, pathogens that contain mannose-rich cell walls activate the complement cascade through the mannose-binding lectin pathway. Mannose-binding lectin binds to distinctive groups of mannose sugars on the surface of the pathogen and then triggers activation of two mannose-binding lectin–associated proteases, MASP-1 and MASP-2, which allow activation of the downstream components of the complement cascade as shown in Figure 23-5. Finally, many microbial surfaces have physical and chemical properties, incompletely understood, that result in activation of complement via the alternative pathway, an activation cascade that includes factors B, D, and P, all proteins found in plasma.
The three pathways converge at the activation of complement protein C3. This protein is synthesized as a precursor that contains an internal, strained thioester linkage between a cysteine and a glutamate residue in close proximity, requiring a proteolytic conversion to become fully reactive. C3 is covalently deposited only on antigen-antibody complexes in close proximity to itself. Surfaces that are properly decorated with mannose-binding lectin or that receive C3 deposits via the alternative pathway are similarly targeted. This proximity restriction limits the effects of complement to nearby surfaces, avoiding an inappropriate attack on cells that do not display the antigens targeted.
Regardless of the activation pathway, activated C3 unleashes the terminal components of the complement cascade, complement proteins C5 through C9, culminating in formation of the membrane attack complex, which inserts itself into almost any adjacent biological membrane and renders it permeable by forming a pore. The resulting loss of electrolytes and small solutes leads to lysis and death of the target cell. Whenever complement is activated, the membrane attack complex is formed and results in death of the cell onto which it is deposited. The direct microbe-killing (microbicidal) effect of a fully activated complement cascade is an important mechanism of host defense.
All three complement activation pathways also generate C3a and C5a cleavage fragments, which bind to G protein–coupled receptors and function to attract neutrophils and other cells involved in inflammation. In addition, phagocytic cells, such as macrophages, which recognize cells whose surfaces are covalently labeled with fragments from C3, ingest and destroy those cells.
The complement cascade thus fulfills multiple roles in host defense: it can destroy the membranes that envelope a pathogen (bacteria, viruses); it covalently “paints” the targeted pathogen so that it may be more readily ingested by phagocytic cells capable of killing the pathogen and presenting its contents to cells that will initiate an adaptive immune response; and finally, the act of complement activation yields signals to attract cells of the innate (neutrophils, macrophages, dendritic cells) and adaptive (lymphocytes) immune systems to the site of infection. These cues are called chemotactic signals.
Natural Killer Cells In addition to bacterial and eukaryotic parasitic invaders, the innate immune system also defends against viruses. When the presence of a virus-infected cell is detected, still other cell types of the innate immune system become active, seeking out virus-infected target cells and killing them. For instance, when many types of cells (not just immune-system cells) are infected, they synthesize and secrete a class of proteins called type I interferons that act as intercellular signals, warning the immune system that an infection is present. The interferons are classified as cytokines, small, secreted proteins that help regulate immune responses in a variety of ways. We will encounter other cytokines and discuss some of their receptors as the chapter progresses.
Interferons activate natural killer (NK) cells. Activated NK cells help protect the body in several ways. First, they can kill host cells infected by a virus (hence the name “natural killer”), preventing those infected cells from making additional virus particles that would spread the infection. Second, NK cells secrete type II interferon γ, which is essential for orchestrating many other aspects of antiviral defenses (Figure 23-6). Third, NK cells can kill target cells that have been decorated by antibodies. NK cells recognize their targets by means of several classes of surface receptors capable of yielding stimulatory (promoting cell killing) or inhibitory signals.
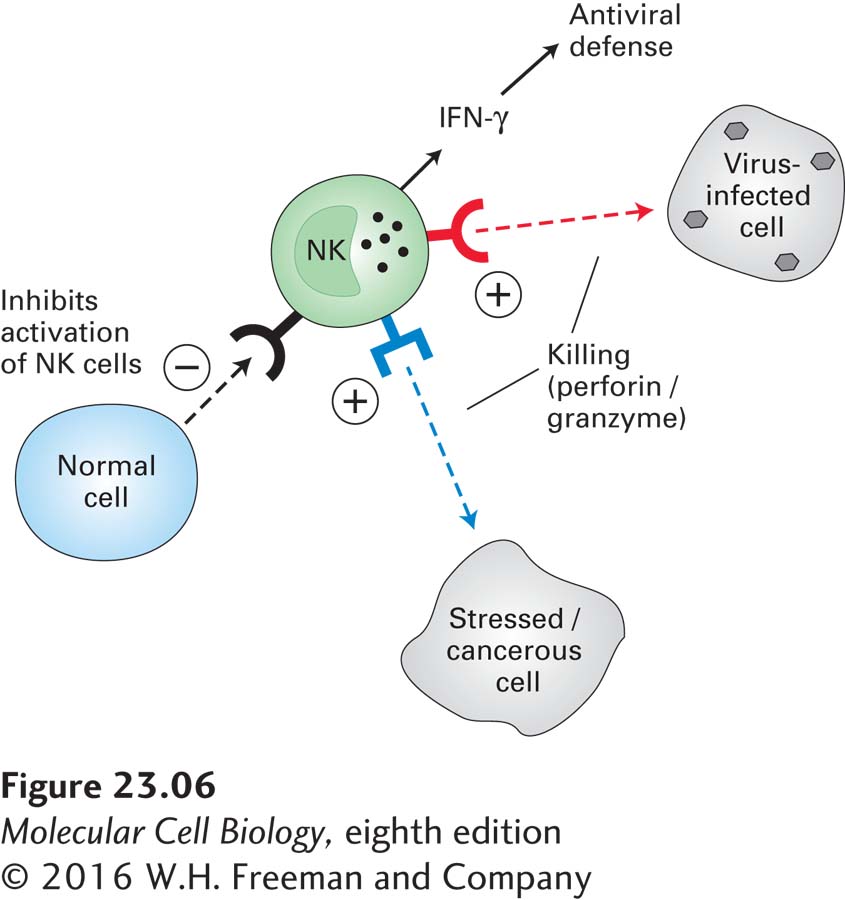
FIGURE 23-6 Natural killer cells. Natural killer (NK) cells are an important source of the cytokine interferon γ (IFN-γ), which is involved in antiviral defenses, and can kill virus-infected and cancerous cells directly by means of perforins. These pore-forming proteins allow access to the cytoplasm of the target cell by serine proteases called granzymes. Granzymes can also initiate apoptosis through activation of caspases (see Chapter 21). Receptors on NK cells identify infected or stressed cells and stimulate the NK cell to kill them. Other receptors identify normal cells and inhibit NK cell activation.


