Many Oncogenes Encode Constitutively Active Signal-Transducing Proteins
A large number of oncogenes are derived from proto-oncogenes whose encoded proteins are components or regulators of signal transduction pathways—most prominent among them the Ras pathway. As we saw in Chapter 16, Ras is a key component in the transduction of signals from activated receptors to a cascade of protein kinases. In the first part of this pathway, a signal from an activated RTK is carried via two adapter proteins to RAS, converting it to the active GTP-bound form (see Figure 16-21). In the second part of the pathway, activated RAS transmits the signal via two intermediate protein kinases to MAP kinase. The activated MAP kinase then phosphorylates a number of transcription factors that induce synthesis of important growth and proliferation proteins (see Figure 16-26). Virtually every component of this RTK/Ras/MAP kinase signaling cascade has been identified as an oncogene or tumor-suppressor gene (Figure 24-21).
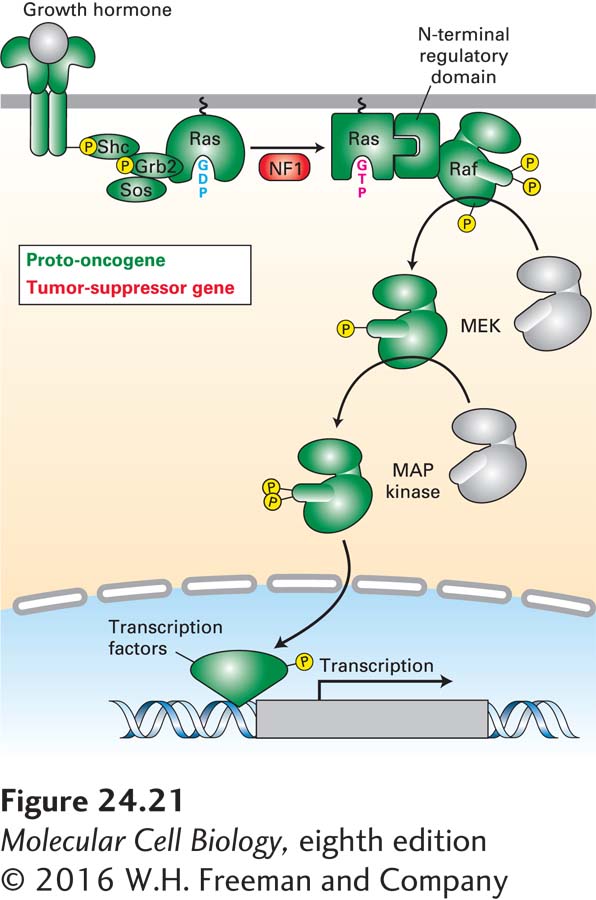
FIGURE 24-21 RTK/RAS/MAP kinase pathway components are frequently mutated in cancer. Components of the RTK/RAS/MAP kinase pathway in which oncogenic mutations have been identified in human cancers are highlighted in green. Components that have been found mutated to cause inactivation of the gene in cancer cells are highlighted in red.
Among the best-studied oncogenes are the rasD genes themselves, which were the first nonviral oncogenes to be recognized (see Classic Experiment 24-1). Any one of a number of changes in RAS can lead to its uncontrolled and therefore dominant activity. In particular, if a point mutation substitutes any other amino acid for the glycine at position 12 in the RAS sequence, the normal protein is converted into a constitutively active oncoprotein (see Chapter 16). This simple mutation reduces the protein’s GTPase activity, thus maintaining RAS in the active GTP-bound state. Activating RAS mutations short-circuit the first part of the RTK pathway, making upstream activation triggered by ligand binding to the receptor unnecessary. Constitutively active RAS oncoproteins are produced by many types of human tumors, including bladder, colon, mammary, skin, and lung carcinomas, neuroblastomas, and leukemias.
Constitutive RAS activation can also arise from a recessive loss-of-function mutation in a GTPase-activating protein (GAP). The normal function of a GAP is to accelerate hydrolysis of GTP and thus the conversion of active GTP-bound Ras to inactive GDP-bound RAS (see Figure 3-34). The loss of GAP leads to sustained RAS activation of downstream signal-transducing proteins. For example, neurofibromatosis, a benign tumor of the sheath cells that surround nerves, is caused by loss of both alleles of NF1, which encodes a RAS GAP-type protein (see Figure 8-20). Individuals with neurofibromatosis have inherited a single mutant NF1 allele; subsequent somatic mutation in the other allele leads to formation of neurofibromas. Thus NF1, like RB, is a tumor-suppressor gene, and the tendency to develop neurofibromatosis, like hereditary retinoblastoma, is inherited as an autosomal dominant trait.
Oncogenes encoding other altered components of the RTK/RAS/MAP kinase pathway have also been identified (see Figure 24-21). For example, constitutively active forms of RAF have been identified in approximately 50 percent of melanomas. As in the case of constitutively active forms of RAS, these mutant RAF forms are no longer responsive to regulatory signals coming from the cell surface and signal continuous cell growth and proliferation.
In addition to RTK/RAS/MAP kinase signaling pathway constituents, cytoplasmic protein kinases are frequently mutated in cancer. Indeed, the first oncogene to be discovered, v-src from Rous sarcoma retrovirus, encodes a constitutively active protein tyrosine kinase. At least eight mammalian proto-oncogenes encode a family of nonreceptor tyrosine kinases related to the SRC protein. In other instances, kinases are fused to other proteins, endowing the protein kinase with new specificity. The BCR-ABL fusion protein is an example of such an oncokinase. As described above, hyperactive kinases and oncokinases have been successfully targeted in cancer therapy.
