Aberrations in Signaling Pathways That Control Development Are Associated with Many Cancers
During normal development, secreted signals such as Hedgehog (Hh), Wnt, and TGF-β are used to direct cells to particular developmental fates, which may include the property of rapid mitosis. The effects of such signals must be regulated so that growth is limited to the right time and place. Among the mechanisms available for reining in the effects of these powerful developmental signals are inducible intracellular antagonists, receptor blockers, and competing signals. Mutations that prevent such restraining mechanisms from operating are likely to be oncogenic, causing inappropriate or cancerous growth.
The Hedgehog signaling pathway, which is used repeatedly during development to control cell fates, is a good example of a signaling pathway implicated in cancer induction. In the skin and cerebellum, one of the human Hh proteins, Sonic Hedgehog, stimulates cell division by binding to and inactivating a membrane protein called Patched1 (PTC1) (see Figure 16-34). Loss-of-function mutations in PTC1 permit cell proliferation in the absence of an Hh signal; thus PTC1 is a tumor-suppressor gene. People who inherit a single working copy of PTC1 have a propensity to develop skin and brain cancer; either can occur when the remaining allele is damaged. Other people can get these diseases too if they lose both copies of the gene. Thus there are both familial and sporadic cases of these diseases, just as in retinoblastoma. Mutations in other genes in the Hh signaling pathway are also associated with cancer. Some such mutations create oncogenes that turn on Hh target genes inappropriately; others are recessive mutations that affect negative regulators such as PTC1. As is the case for a number of other tumor-suppressor genes, complete loss of PTC1 function would lead to early fetal death, since the protein is needed for development, so it is only the tumor cells that are homozygous (ptc1/ptc1).
Many of the signaling pathways described in other chapters also play roles in controlling embryonic development and cell proliferation in adult tissues. In recent years, mutations affecting components of most of these signaling pathways have been linked to cancer. Indeed, once one gene in a developmental pathway has been linked to a type of human cancer, knowledge of that pathway gleaned from model organisms such as worms, flies, or mice allows focused investigations of the possible involvement of additional pathway genes in other cases of the cancer. For example, APC, a gene that is mutated on the path to colon cancer, is now known to be part of the Wnt signaling pathway (see Chapter 16). That knowledge, in turn, led to the discovery of β-catenin mutations in colon cancer.
Mutations in tumor-suppressor developmental genes promote tumor formation in tissues where the affected gene normally helps restrain growth. Thus these mutations do not cause cancers in tissues where the primary role of the developmental regulator is to control cell fate—what type of cell develops—but not cell division. Mutations in developmental proto-oncogenes may induce tumor formation in tissues where an affected gene normally promotes cell proliferation or in another tissue where the gene has become aberrantly active.
Transforming growth factor β (TGF-β), despite its name, inhibits proliferation of many cell types, including most epithelial and immune-system cells. Binding of TGF-β to its receptor activates cytosolic Smad transcription factors (see Figure 16-3). After translocating to the nucleus, Smads can promote expression of the gene encoding p15, an inhibitor of cyclin-dependent kinase 4 (CDK4), which causes cells to arrest in G1. TGF-β signaling also promotes expression of genes encoding extracellular matrix proteins and plasminogen activator inhibitor 1 (PAI-1), which reduces the plasmin-catalyzed degradation of the matrix. Loss-of-function mutations in either TGF-β receptors or in Smads thus promote cell proliferation and probably contribute to the invasiveness and metastasis of tumor cells (Figure 24-24). Such mutations have in fact been found in a variety of human cancers. For example, deletion of the Smad4 gene occurs in many human pancreatic cancers; retinoblastoma and colon cancer cells lack functional TGF-β receptors and therefore are unresponsive to TGF-β growth inhibition.
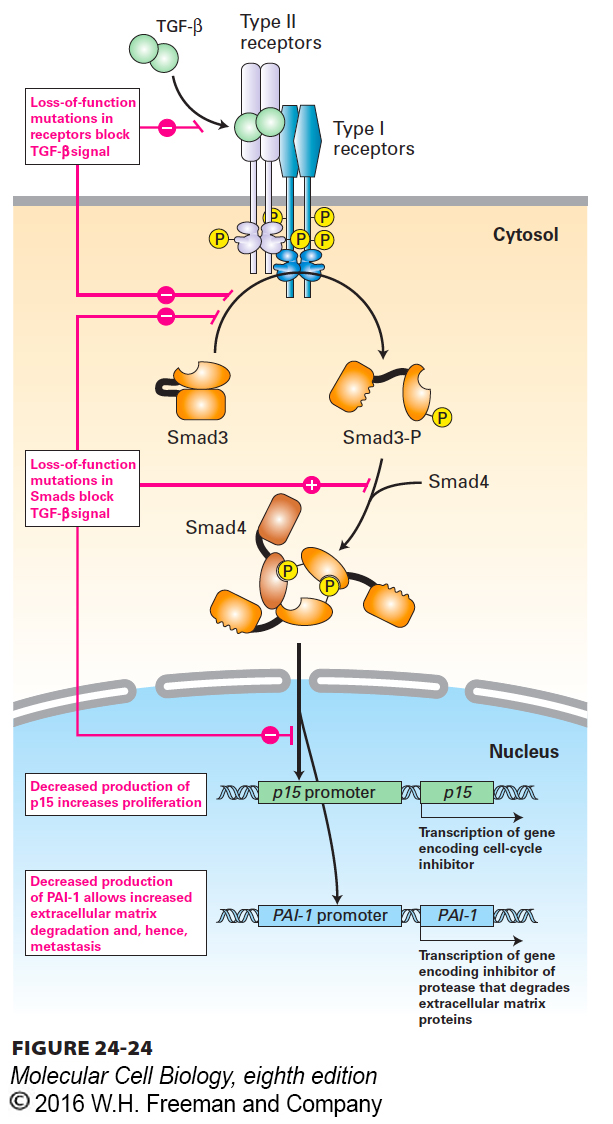
FIGURE 24-24 Effect of loss of TGF-β signaling. Binding of TGF-β, an anti-growth factor, to its receptor causes activation of Smad transcription factors. In the absence of effective TGF-β signaling owing to either a receptor mutation or a Smad mutation, cell proliferation and invasion of the surrounding extracellular matrix increase. See X. Hua et al., 1998, Genes & Dev. 12:3084.
