Cellular Housekeeping Functions Are Fundamentally Altered in Cancer Cells
Cancer cells can often be distinguished from normal cells by microscopic examination. They are usually less well differentiated than normal cells. Cancer cells frequently exhibit the characteristics of rapidly growing cells: a high nucleus-to-cytoplasm ratio, prominent nucleoli, an increased frequency of mitotic cells, and relatively little specialized structure.
Tumor cells differ from normal cells not only in their appearance, but in their entire protein composition. Genome-wide gene expression and protein composition analyses have shown that in all organisms studied to date, increases in gene copy number lead to corresponding increases in gene expression. (Notable exceptions to this observation are the genes located on sex chromosomes.) Thus the genomic changes so characteristic of cancer cells—losses and gains of whole chromosomes or chromosome parts—have a profound effect on the protein composition of the cell and hence on most, if not all, cellular functions. This, in turn, causes a stress response that is aimed at offsetting the protein imbalances that cancer cells experience. For example, cancer cells rely heavily on protein folding and degradation mechanisms for their survival as a direct result of their dramatically altered chromosome composition. This dependence of cancer cells on proteome maintenance pathways for their survival makes the constituents of these pathways attractive drug targets.
Another prominent feature of cancer cells is their use of an unusual energy-generating mechanism. Normal differentiated cells rely on mitochondrial oxidative phosphorylation to satisfy their energy needs. Cells metabolize glucose to carbon dioxide by oxidation of pyruvate through the tricarboxylic acid (TCA) cycle in the mitochondria (see Chapter 12). Only under anaerobic conditions do normal cells undergo anaerobic glycolysis and produce large amounts of lactate. Most cancer cells, however, rely on glycolysis for energy production irrespective of whether oxygen levels are high or low (Figure 24-3). The use of glycolysis to produce energy even in the presence of oxygen, called aerobic glycolysis, was first discovered in cancer cells by the biochemist Otto Warburg and is therefore called the Warburg effect. The metabolism of glucose to lactate generates only 2 ATP molecules per molecule of glucose, while oxidative phosphorylation generates up to 36 molecules of ATP per molecule of glucose. Cells can, however, use glycolysis intermediates to synthesize macromolecules and lipids. The rewiring of glucose metabolism could thus provide the fuel necessary to sustain macromolecule biosynthesis and proliferation of cancer cells.
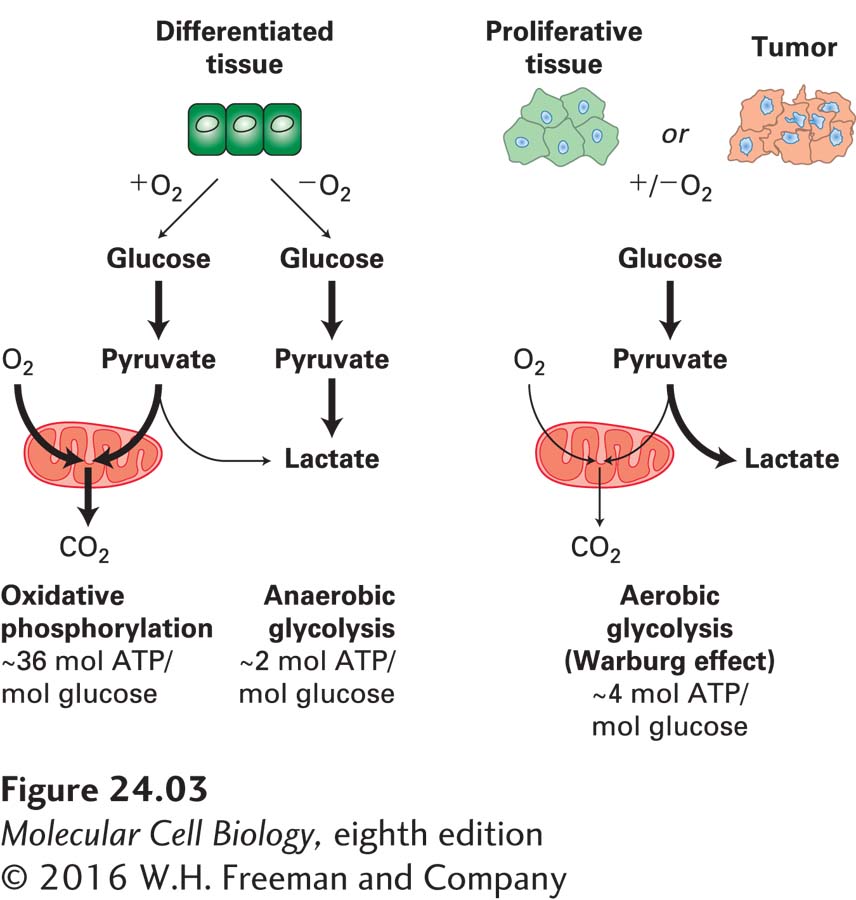
FIGURE 24-3 Energy production in cancer cells by aerobic glycolysis. In the presence of oxygen, nonproliferating (differentiated) cells metabolize glucose into pyruvate via glycolysis. Pyruvate is then transported into mitochondria, where it is fed into the TCA cycle. Oxygen is required as the final electron acceptor during oxidative phosphorylation. Thus, when oxygen is limiting, cells metabolize pyruvate into lactate, allowing glycolysis to continue by cycling NADH back to NAD+. Cancer cells and proliferating cells convert most glucose to lactate regardless of whether oxygen is present or not. The production of lactate in the presence of oxygen is called aerobic glycolysis. See M. G. Vander Heiden et al., 2009, Science 324:1029.
Not only do cancer cells rewire their metabolic pathways, but some cancer types produce novel metabolites that play a critical role in the disease. Seventy percent of glioblastomas, oligodendrogliomas, and astrocytomas (all brain cancers) and approximately 25 percent of acute myeloid leukemias harbor mutations in isocitrate dehydrogenase (IDH), a TCA cycle enzyme that converts isocitrate to α-ketoglutarate (Figure 24-4). The IDH mutations found in these cancers cause the enzyme to convert isocitrate into a new metabolite, 2-hydroxyglutarate, which accumulates to levels of up to 5–35 mM in cancer cells! So how does 2-hydroxyglutarate promote tumorigenesis? It inhibits several enzymes that require α-ketoglutarate for their function, including proteins that regulate the methylation state of histones. In this way, 2-hydroxyglutarate alters gene expression. Whether 2-hydroxyglutarate is the only example of a cancer-specific metabolite or whether it is but the first in a new class of oncometabolites remains to be seen.
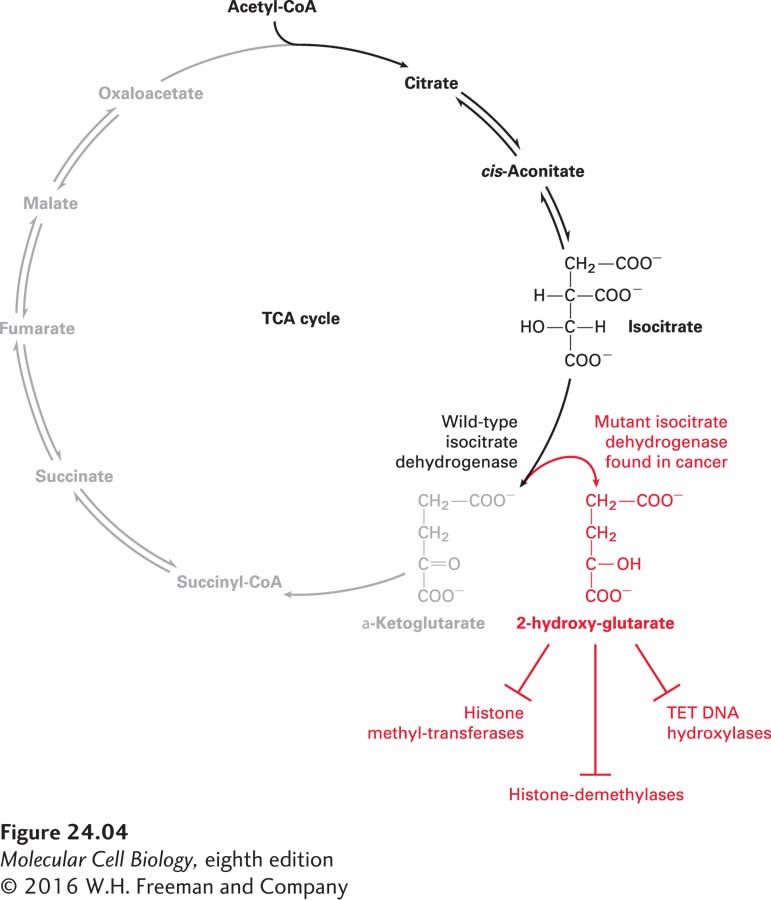
FIGURE 24-4 2-Hydroxyglutarate is a cancer-specific metabolite. Isocitrate dehydrogenase (IDH) is an enzyme in the TCA cycle. It converts isocitrate into α-ketoglutarate, which is then further converted into succinyl CoA. Some mutant forms of IDH found in many brain cancers create a novel metabolite, 2-hydroxyglutarate (2HG), from α-ketoglutarate. 2-Hydroxyglutarate accumulates to extremely high levels in these cells and inhibits the activity of proteins that require α-ketoglutarate for their function. Many of the enzymes that are inhibited control gene expression, such as the TET family of DNA hydroxylases, or the methylation state of histones, such as histone methyl transferases and demethylases.

