Invasion and Metastasis Are Late Stages of Tumorigenesis
Tumors arise with great frequency, especially in older individuals, but most pose little risk to their host because they are small and localized. We call such tumors benign; an example is a wart, a benign skin tumor. The cells composing benign tumors closely resemble, and may function like, normal cells. The cell-adhesion molecules that hold tissues together keep benign tumor cells, like normal cells, localized to the tissues where they originate. A fibrous capsule usually delineates the extent of a benign tumor and makes it an easy target for a surgeon. Benign tumors become serious medical problems only if their sheer bulk interferes with normal functions or if they secrete excess amounts of biologically active substances such as hormones. For example, acromegaly, the overgrowth of head, hands, and feet, can occur when a benign pituitary tumor causes overproduction of growth hormone.
In contrast to benign tumor cells, malignant tumor cells are able to invade nearby tissue, spreading and seeding additional tumors while the cells continue to proliferate (Figure 24-6). This ability is a major characteristic that differentiates malignant tumors from benign ones. Some malignant tumors, such as those in the ovary or breast, remain localized and encapsulated, at least for a time. When these tumors progress, however, the cells invade surrounding tissues and undergo metastasis (Figure 24-7a).
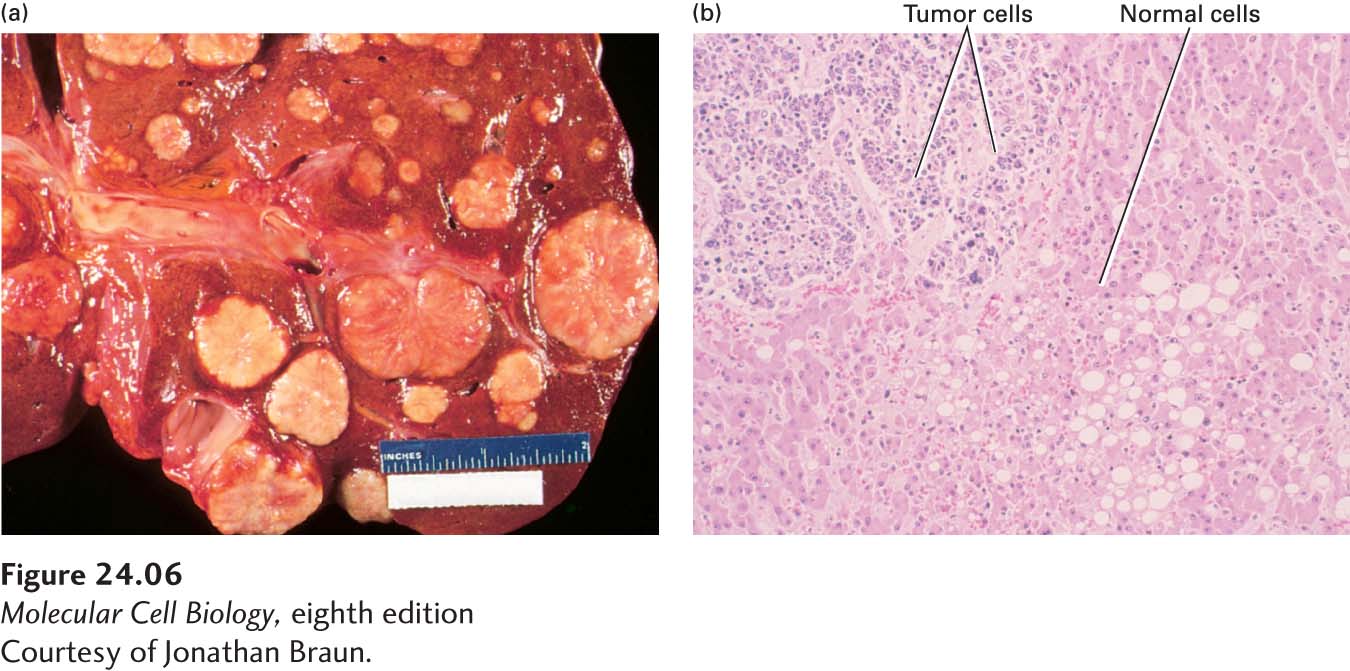
FIGURE 24-6 Gross and microscopic views of a tumor invading normal liver tissue. (a) The gross morphology of a human liver in which a metastatic lung tumor is growing. The white protrusions on the surface of the liver are the tumor masses. (b) A light micrograph of a section of the tumor in (a), showing areas of small, dark-staining tumor cells invading a region of larger, light-staining, normal liver cells.
[Courtesy of Jonathan Braun.]
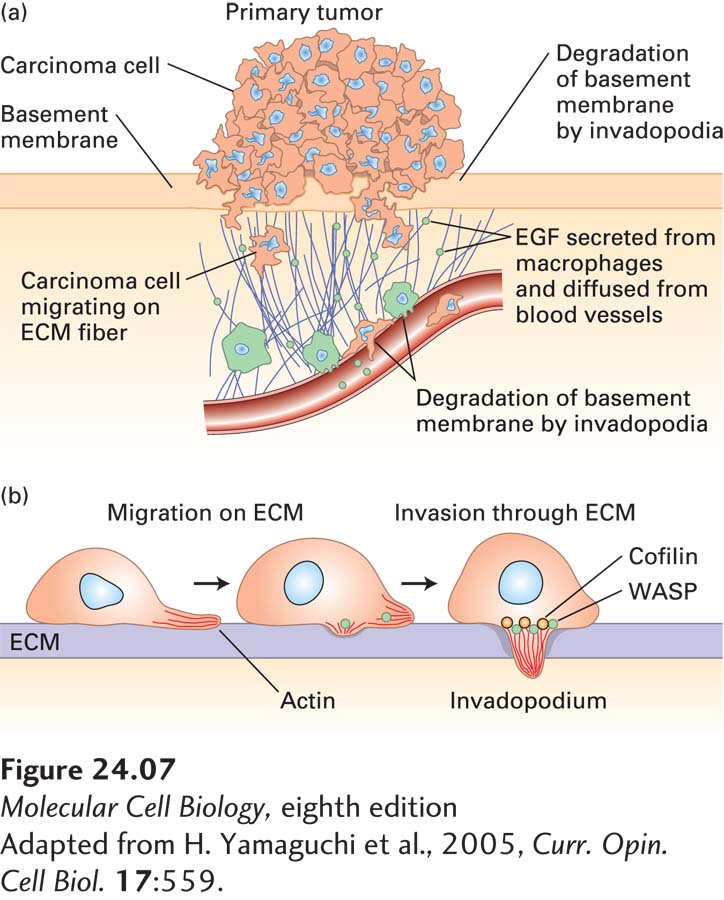
FIGURE 24-7 Metastasis. (a) First steps in metastasis, using breast carcinoma cells as an example. Cancer cells leave the main tumor and attack the basement membrane, using extracellular matrix (ECM) fibers to reach the blood vessels. The cancer cells can be attracted by signals such as epidermal growth factor (EGF), which can be secreted by macrophages. At the blood vessels they penetrate the layer of endothelial cells that forms the vessel walls and enter the bloodstream. (b) Carcinoma cells penetrate the extracellular matrix and blood vessel wall by extending “invadopodia,” which produce matrix metalloproteases and other proteases to open up a path.
[Adapted from H. Yamaguchi et al., 2005, Curr. Opin. Cell Biol. 17:559.]
Normal cells are restricted to their place in an organ or tissue by cell-cell adhesion and by physical barriers such as the basement membrane, which underlies layers of epithelial cells and also surrounds the endothelial cells of blood vessels (see Chapter 20). In contrast, cancer cells have acquired the ability to penetrate basement membranes using a cell protrusion called an invadopodium and to migrate to distant sites in the body (Figure 24-7b). A developmental process known as the epithelial-to-mesenchymal transition (EMT) is thought to play a crucial role during the process of metastasis in some cancers. During normal development, the conversion of epithelial cells into mesenchymal cells is a step in the formation of some organs and tissues. An EMT requires distinct changes in patterns of gene expression and results in fundamental changes in cell morphology, such as loss of cell-cell adhesion, loss of cell polarity, and the acquisition of migratory and invasive properties. During metastasis, the EMT regulatory pathways are thought to be activated at the invasive front of a tumor, producing single migratory cells. At the heart of the EMT are two transcription factors, Snail and Twist. These transcription factors promote expression of genes involved in cell migration, trigger down-regulation of cell-adhesion factors such as E-cadherin, and increase the production of proteases that digest the basement membrane, thus allowing its penetration by the tumor cells. For example, many tumor cells secrete a protein (plasminogen activator) that converts the serum protein plasminogen to the active protease plasmin. Importantly, expression of many important drivers of the EMT, such as SNAIL1 and SNAIL2, has been shown to correlate with disease relapse and decreased patient survival in many cancers, including breast, colon, and ovarian cancer. The occurrence of the EMT predicts a poor clinical outcome.
As the basement membrane disintegrates, some tumor cells enter the bloodstream, but fewer than 1 in 10,000 cells that escape the primary tumor survive to colonize another tissue and form a secondary, metastatic tumor. Much of preventative medicine is currently focused on developing methods to identify the rare tumor cells that circulate in the bloodstream. The ability to capture these circulating tumor cells would not only provide a powerful and noninvasive tool for the early detection of cancer, but their analysis could provide insights into the nature of the disease and inform treatment.
In order to produce metastases, tumor cells must not only enter the bloodstream, but adhere to the lining of the blood vessel in a new location and migrate through it into the underlying tissue in a process called extravasation (see Chapter 20). In order to seed a metastasis at a distant site, the tumor cells must not only disseminate, but also adapt to a foreign tissue environment. At least initially, metastatic tumor cells may not be well adapted to their new environment, but they are thought to evolve to survive and thrive in a foreign context. Little is known about the molecular pathways that facilitate this adaptation, but mounting evidence suggests that some environments are more conductive to cancer cell colonization than others.
Because metastasis is the most common reason for morbidity associated with cancer, much effort is being put into understanding which tumors will become metastatic and how metastasis occurs. Traditionally, the properties of tumor and normal cells have been assessed using microscopic tools, and the prognosis for many tumors could be determined, within certain limits, from their histology. However, the appearance of cells alone has limited information content, and better ways to discern the properties of cells are desirable, both to understand tumorigenesis and to arrive at meaningful and accurate decisions about prognosis and therapy. The advent of methods to assess a tumor’s patterns of RNA, protein, lipid, and metabolite production is allowing for a more detailed examination of tumor properties. Not surprisingly, primary tumors are often distinguishable from metastatic tumors by the RNAs and proteins that they produce. Analyses of global patterns of gene expression (described in Chapter 6) are now routinely used to predict patient outcomes and to determine the best course of treatment for many types of cancers, and they will soon become the standard in determining treatment options.

