Monosaccharides Covalently Assemble into Linear and Branched Polysaccharides
The building blocks of the polysaccharides are the simple sugars, or monosaccharides. Monosaccharides are carbohydrates, which are literally covalently bonded combinations of carbon and water in a one-
47
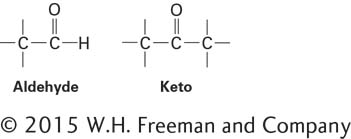
Many biologically important sugars are hexoses, including glucose, mannose, and galactose (Figure 2-18). Mannose is identical to glucose except that the orientation of the groups bonded to carbon 2 is reversed. Similarly, galactose, another hexose, differs from glucose only in the orientation of the groups attached to carbon 4. Interconversion of glucose and mannose or galactose requires the breaking and making of covalent bonds; such reactions are carried out by enzymes called epimerases.
D-Glucose (C6H12O6) is the principal external source of energy for most cells in complex multicellular organisms. It can exist in three different forms: a linear structure and two different hemiacetal ring structures (Figure 2-18a). If the aldehyde group on carbon 1 combines with the hydroxyl group on carbon 5, the resulting hemiacetal, D-glucopyranose, contains a six-
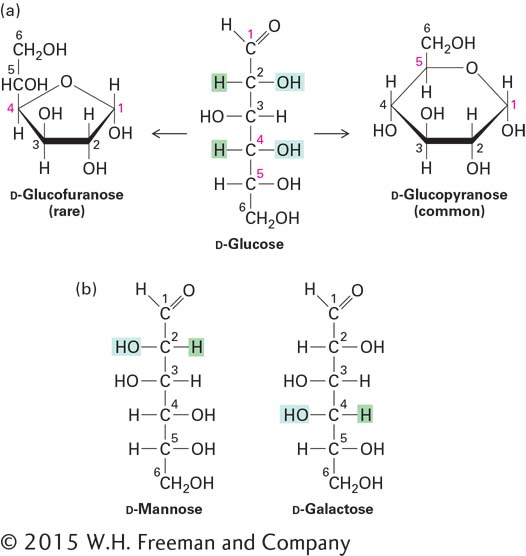
The pyranose ring in Figure 2-18a is depicted as planar. In fact, because of the tetrahedral geometry around carbon atoms, the most stable conformation of a pyranose ring has a nonplanar, chairlike shape. In this conformation, each bond from a ring carbon to a nonring atom (e.g., H or O) is either nearly perpendicular to the ring, referred to as axial (a), or nearly in the plane of the ring, referred to as equatorial (e):
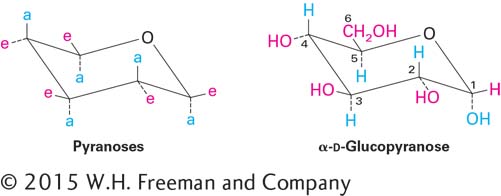
Disaccharides, formed from two monosaccharides, are the simplest polysaccharides. The disaccharide lactose, composed of galactose and glucose, is the major sugar in milk; the disaccharide sucrose, composed of glucose and fructose, is a principal product of plant photosynthesis and is refined into common table sugar (Figure 2-19).
Larger polysaccharides, containing dozens to hundreds of monosaccharide units, can function as reservoirs for glucose, as structural components, or as adhesives that help hold cells together in tissues. The most common storage carbohydrate in animal cells is glycogen, a very long, highly branched polymer of glucose. As much as 10 percent of the liver by weight can be glycogen. The primary storage carbohydrate in plant cells, starch, is also a glucose polymer. It occurs in an unbranched form (amylose) and a lightly branched form (amylopectin). Both glycogen and starch are composed of the α anomer of glucose. In contrast, cellulose, the major constituent of plant cell walls, which confers stiffness to many plant structures (see Chapter 19), is an unbranched polymer of the β anomer of glucose. Human digestive enzymes can hydrolyze the α glycosidic bonds in starch but not the β glycosidic bonds in cellulose. Many species of plants, bacteria, and molds produce cellulose-
48
The enzymes that make the glycosidic bonds linking monosaccharides into polysaccharides are specific for the α or β anomer of one sugar and a particular hydroxyl group on the other. In principle, any two sugar molecules can be linked in a variety of ways because each monosaccharide has multiple hydroxyl groups that can participate in the formation of glycosidic bonds. Furthermore, any one monosaccharide has the potential to be linked to more than two other monosaccharides, thus generating a branch point and nonlinear polymers. Glycosidic bonds are usually formed between the growing polysaccharide chain and a covalently modified form of a monosaccharide. Such modifications include the addition of a phosphate (e.g., glucose-
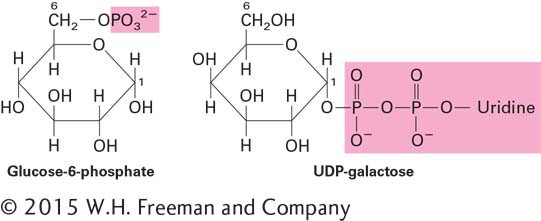
The epimerase enzymes that interconvert different monosaccharides often do so using the nucleotide sugars rather than the unmodified, or “free,” sugars.
Many complex polysaccharides contain modified sugars that are covalently linked to various small groups, particularly amino, sulfate, and acetyl groups. Such modifications are abundant in glycosaminoglycans, major polysaccharide components of the extracellular matrix that we describe in Chapter 19.