A Chemical Reaction Is in Equilibrium When the Rates of the Forward and Reverse Reactions Are Equal
When reactants first mix together—before any products have been formed—the rate of the forward reaction to form products is determined in part by the reactants’ initial concentrations, which determine the likelihood of reactants bumping into one another and reacting (Figure 2-22). As the reaction products accumulate, the concentration of each reactant decreases, and so does the forward reaction rate. Meanwhile, some of the product molecules begin to participate in the reverse reaction, which re-forms the reactants. The ability of a reaction to go “backward” is called microscopic reversibility. The reverse reaction is slow at first but speeds up as the concentration of product increases. Eventually, the rates of the forward and reverse reactions become equal, so that the concentrations of reactants and products stop changing. The system is then said to be in chemical equilibrium (plural, equilibria).
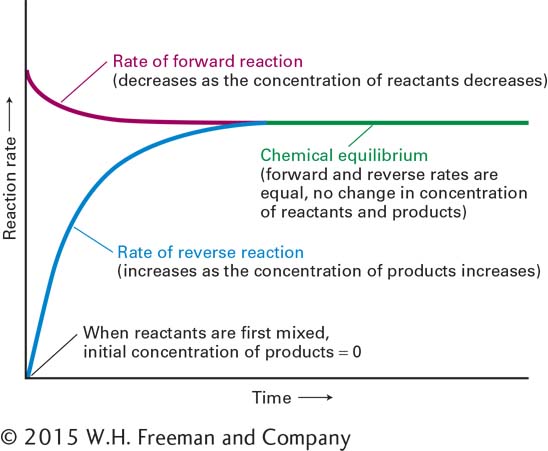
FIGURE 2-22 Time dependence of the rates of a chemical reaction. The forward and reverse rates of a reaction depend in part on the initial concentrations of reactants and products. The net forward reaction rate slows as the concentration of reactants decreases, whereas the net reverse reaction rate increases as the concentration of products increases. At equilibrium, the rates of the forward and reverse reactions are equal, and the concentrations of reactants and products remain constant.
The ratio of the concentrations of the products to the concentrations of the reactants when they reach equilibrium, called the equilibrium constant (Keq), is a fixed value. Thus Keq provides a measure of the extent to which a reaction occurs by the time it reaches equilibrium. The rate of a chemical reaction can be increased by a catalyst, but a catalyst does not change the equilibrium constant (see Section 2.4). A catalyst accelerates the making and breaking of covalent bonds but itself is not permanently changed during a reaction.
