Life Depends on the Coupling of Unfavorable Chemical Reactions with Energetically Favorable Ones
Many processes in cells are energetically unfavorable (ΔG > 0) and will not proceed spontaneously. Examples include the synthesis of DNA from nucleotides and the transport of a substance across the plasma membrane from a lower to a higher concentration. Cells can carry out an energy-
Suppose, for example, that the reaction A ⇌ B + X has a ΔG of +5 kcal/mol and that the reaction X ⇌ Y + Z has a ΔG of –10 kcal/mol:
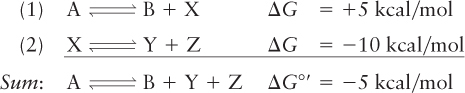
In the absence of the second reaction, there would be much more A than B at equilibrium. However, because the conversion of X to Y + Z is such a favorable reaction, it will pull the first process toward the formation of B and the consumption of A. Energetically unfavorable reactions in cells are often coupled to the energy-