NAD+ and FAD Couple Many Biological Oxidation and Reduction Reactions
In many chemical reactions, electrons are transferred from one atom or molecule to another; this transfer may or may not accompany the formation of new chemical bonds or the release of energy that can be coupled to other reactions. The loss of electrons from an atom or a molecule is called oxidation, and the gain of electrons by an atom or a molecule is called reduction. An example of oxidation is the removal of electrons from the sulfhydryl group–
2 Fe2+ + ½ O2 → 2 Fe2+ + O2–
Thus Fe2+ is oxidized and O2 is reduced. Such reactions in which one molecule is reduced and another is oxidized are often referred to as redox reactions. Oxygen is an electron acceptor in many redox reactions in cells under aerobic conditions.
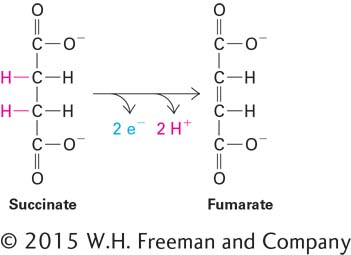
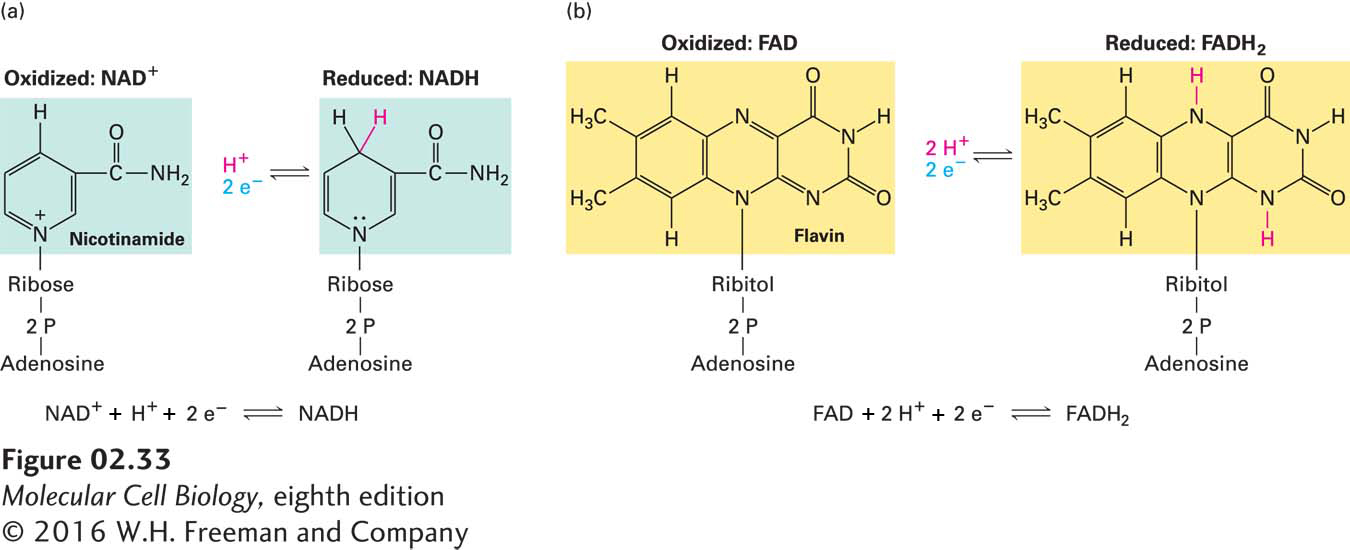
Many biologically important oxidation and reduction reactions involve the removal or addition of hydrogen atoms (protons plus electrons) rather than the transfer of isolated electrons on their own. The oxidation of succinate to fumarate, which occurs in mitochondria, is an example (Figure 2-32). Protons are soluble in aqueous solutions (as H3O+), but electrons are not, so they must be transferred directly from one atom or molecule to another without a water-
To describe redox reactions, such as the reaction of ferrous ion (Fe2+) and oxygen (O2), it is easiest to divide them into two half-
Oxidation of Fe2+: 2 Fe2+ → 2 Fe3+ + 2 e–
Reduction of O2: 2 e– + ½ O2 → O2–
64
In this case, the reduced oxygen (O2–
Reduction potentials are measured in volts (V) from an arbitrary zero point set at the reduction potential of the following half-
The value of E for a molecule or an atom under standard conditions is its standard reduction potential, E′0. A molecule or an ion with a positive E′0 has a higher affinity for electrons than the H+ ion does under standard conditions. Conversely, a molecule or ion with a negative E′0 has a lower affinity for electrons than the H+ ion does under standard conditions. Like the values of ΔG°′, standard reduction potentials may differ somewhat from those found under the conditions in a cell because the concentrations of reactants in a cell are not 1 M.
In a redox reaction, electrons move spontaneously toward atoms or molecules having more positive reduction potentials. In other words, a molecule having a more negative reduction potential can transfer electrons spontaneously to, or reduce, a molecule with a more positive reduction potential. In this type of reaction, the change in electric potential ΔE is the sum of the reduction and oxidation potentials for the two half-
ΔG (cal/mol) = –n (23, 064) ΔE (volts) (2-
where n is the number of electrons transferred. Note that a redox reaction with a positive ΔE value will have a negative ΔG and thus will tend to proceed spontaneously from left to right.