Electrons May Be Shared Equally or Unequally in Covalent Bonds
The extent of an atom’s ability to attract an electron is called its electronegativity. In a bond between atoms with identical or similar electronegativities, the bonding electrons are essentially shared equally between the two atoms, as is the case for most carbon-carbon single bonds (C–C) and carbon-hydrogen single bonds (C–H). Such bonds are called nonpolar. In many molecules, however, the bonded atoms have different electronegativities, resulting in unequal sharing of electrons. The bond between them is said to be polar.
One end of a polar bond has a partial negative charge (δ−), and the other end has a partial positive charge (δ+). In an O–H bond, for example, the greater electronegativity of the oxygen atom relative to the hydrogen atom results in the electrons spending more time around the oxygen atom than around the hydrogen. Thus the O–H bond possesses an electric dipole, a positive charge separated from an equal but opposite negative charge. The amount of δ+ charge on the oxygen atom of an O–H dipole is approximately 25 percent that of an electron, and there is an equivalent and opposite δ+ charge on the H atom. A common quantitative measure of the extent of charge separation, or strength, of a dipole is called the dipole moment, µ, which for a chemical bond is the product of the partial charge on each atom and the distance between the two atoms. For a molecule with multiple dipoles, the amount of charge separation for the molecule as a whole depends in part on the dipole moments of all of its individual chemical bonds and in part on the geometry of the molecule (the relative orientations of the individual dipole moments).
Consider the example of water (H2O), which has two O–H bonds and thus two individual bond dipole moments. If water were a linear molecule with the two bonds on exact opposite sides of the O atom, the two dipoles on each end of the molecule would be identical in strength but would be oriented in opposite directions. The two dipole moments would cancel each other, and the dipole moment of molecule as a whole would be zero. However, because water is a V-shaped molecule, with the individual dipoles of its two O–H bonds both pointing toward the oxygen, one end of the water molecule (the end with the oxygen atom) has a partial negative charge and the other end (the one with the two hydrogen atoms) has a partial positive charge. As a consequence, the molecule as a whole is a dipole with a well-defined dipole moment (Figure 2-5). This dipole moment and the electronic properties of the oxygen and hydrogen atoms allow water to form electrostatic, noncovalent interactions with other water molecules and with molecules of other types. These interactions play a critical role in almost every biochemical interaction in cells and organisms, as we will see shortly.
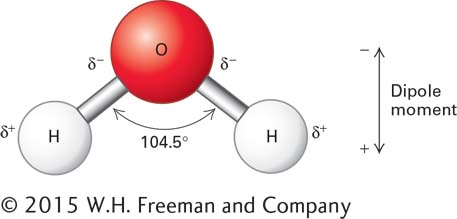
FIGURE 2-5 The dipole nature of a water molecule. The symbol δ represents a partial charge (a weaker charge than the one on an electron or a proton). Because of the difference in the electronegativities of H and O, each of the polar H–O bonds in water is a dipole. The sizes and directions of the dipoles of each of the bonds determine the net distance and amount of charge separation, or dipole moment, of the molecule.
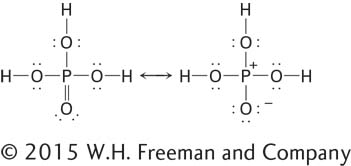
Another important example of polarity is the O=P double bond in H3PO4. In the structure of H3PO4 shown on the left below, lines represent single and double bonds and nonbonding electrons are shown as pairs of dots (each dot represents one electron):
Because of the polarity of the O=P double bond, H3PO4 can also be represented by the structure on the right, in which one of the electrons from the P=O double bond has accumulated around the O atom, giving it a negative charge and leaving the P atom with a positive charge. These charges are important in noncovalent interactions. Neither of these two models precisely describes the electronic state of H3PO4. The actual structure can be considered to be an intermediate, or hybrid, between these two representations, as indicated by the double-headed arrow between them. Such intermediate structures are called resonance hybrids.