Specific Binding of Ligands Underlies the Functions of Most Proteins
The molecule to which a protein binds is called its ligand. In some cases, ligand binding causes a change in the shape of a protein. Such conformational changes are integral to the mechanism of action of many proteins and are important in regulating protein activity.
Two properties of a protein characterize how it binds ligands. Specificity refers to the ability of a protein to bind one molecule or a very small group of molecules in preference to all other molecules. Affinity refers to the tightness or strength of binding, usually expressed as the dissociation constant (Kd). The Kd for a protein-ligand complex, which is the inverse of the equilibrium constant Keq for the binding reaction, is the most common quantitative measure of affinity (see Chapter 2). The stronger the interaction between a protein and ligand, the lower the value of Kd. Both the specificity and the affinity of a protein for a ligand depend on the structure of the ligand-binding site. For high-affinity and highly specific interactions to take place, the shape and chemical properties of the binding site must be complementary to those of the ligand molecule, a property termed molecular complementarity. As we saw in Chapter 2, molecular complementarity allows molecules to form multiple noncovalent interactions at close range and thus stick together.
One of the best-studied examples of protein-ligand binding, involving high affinity and exquisite specificity, is the binding of antibodies to antigens. Antibodies are proteins that circulate in the blood and are made by the immune system in response to antigens, which are usually macromolecules present in infectious agents (e.g., a bacterium or a virus) or other foreign substances (e.g., proteins or polysaccharides in pollens). Different antibodies are generated in response to different antigens, and these antibodies have the remarkable characteristic of binding specifically to (“recognizing”) the part of the antigen, called an epitope, that initially induced the production of the antibody, and not to other molecules. Antibodies act as specific sensors for antigens, forming antibody-antigen complexes that initiate a cascade of protective reactions in cells of the immune system. Chapter 23 discusses antibodies and their roles in the immune system, and later in this chapter we will discuss techniques for studying proteins that exploit antibodies. Here we briefly introduce the structure of antibodies and their binding to epitopes.
Antibodies are Y-shaped molecules, often formed from two identical longer, or heavy, chains and two identical shorter, or light, chains. In IgG antibodies (also called immunoglobulins, shown in Figure 3-21a), there are four globular domains in each heavy chain and two in each light chain, all of which are called immunoglobulin (Ig) domains. Each of the two branching arms of an IgG antibody contains a single light chain linked to a heavy chain by a disulfide bond, and two disulfide bonds covalently link the two heavy chains together. Near the end of each arm are six highly variable loops, called complementarity-determining regions (CDRs), which form the antigen-binding sites. The sequences of the six loops are highly variable among antibodies, generating unique complementary ligand-binding sites that make them specific for different epitopes (Figure 3-21b). The intimate contact between antibody and epitope surfaces, stabilized by numerous noncovalent interactions, is responsible for the extremely precise binding specificity exhibited by an antibody.
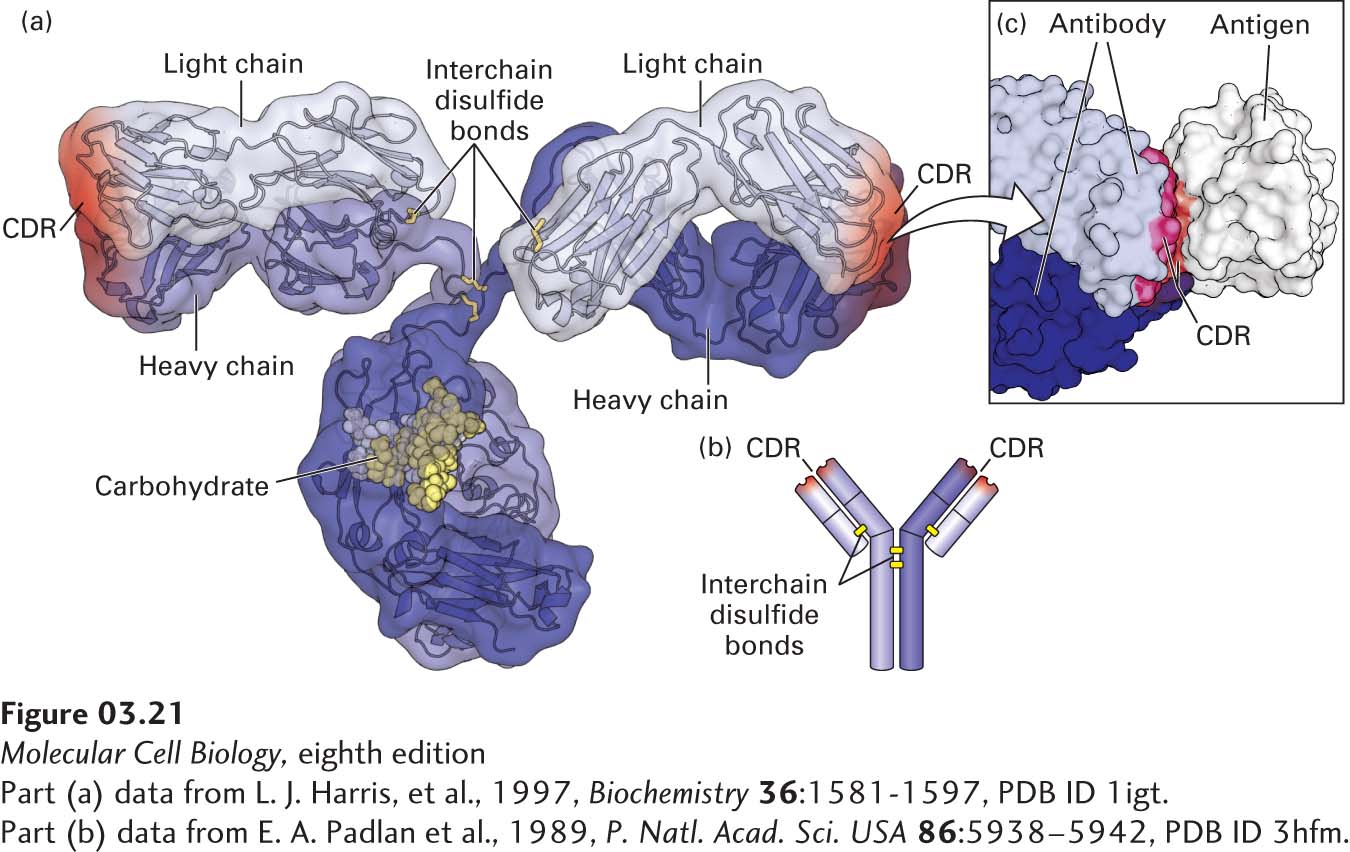
FIGURE 3-21 Protein-ligand binding of antibodies. (a) Hybrid (surface and ribbon) model of an antibody. Every antibody molecule of the immunoglobulin G (IgG) class consists of two identical heavy chains (medium and dark blue) and two identical light chains (light blue) covalently linked by disulfide bonds (yellow). The complementarity-determining regions (CDRs) that define the antigen-binding sites are represented by red shading. (b) The cartoon shows the overall structure containing the two heavy (longer) and two light (shorter) chains, with yellow bars representing disulfide bonds. (c) The hand-in-glove fit between an antibody and the site to which it binds (epitope) on its target antigen—in this case, chicken egg-white lysozyme. The antibody contacts the antigen with residues from its CDRs.
[Part (a) data from L. J. Harris, et al., 1997, Biochemistry 36:1581-1597, PDB ID 1igt. Part (c) data from E. A. Padlan et al., 1989, P. Natl. Acad. Sci. USA 86:5938–5942, PDB ID 3hfm.]
The specificity of antibodies is so precise that they can distinguish between the cells of individual members of a species and in some cases between proteins that differ by only a single amino acid, or even between proteins with identical sequences that differ only in their post-translational modifications. Because of their specificity and the ease with which they can be produced (see Chapter 23), antibodies are highly useful reagents used in many of the experiments discussed in subsequent chapters.
We will see many examples of protein-ligand binding throughout this book, including binding of hormones to receptors (see Chapter 15), binding of regulatory molecules to DNA (see Chapter 9), and binding of cell-adhesion molecules to extracellular matrices (see Chapter 20), to name just a few. Here we focus on how the binding of one class of proteins, enzymes, to their ligands results in the catalysis of the chemical reactions essential for the survival and function of cells.
