Enzymes Are Highly Efficient and Specific Catalysts
90
Proteins that catalyze chemical reactions—
Thousands of different types of enzymes, each of which catalyzes a single chemical reaction or a set of closely related reactions, have been identified. Certain enzymes are found in the majority of cells because they catalyze the synthesis of common cellular products (e.g., proteins, nucleic acids, and phospholipids) or take part in harvesting energy from nutrients (e.g., by the conversion of glucose and oxygen into carbon dioxide and water during cellular respiration). Other enzymes are present only in a particular type of cell because they catalyze chemical reactions unique to that cell type (e.g., the enzymes in neurons that convert tyrosine into dopamine, a neurotransmitter). Although most enzymes are located within cells, some are secreted and function at extracellular sites, such as the blood, the digestive tract, or even outside the organism (e.g., toxic enzymes in the venom of poisonous snakes).
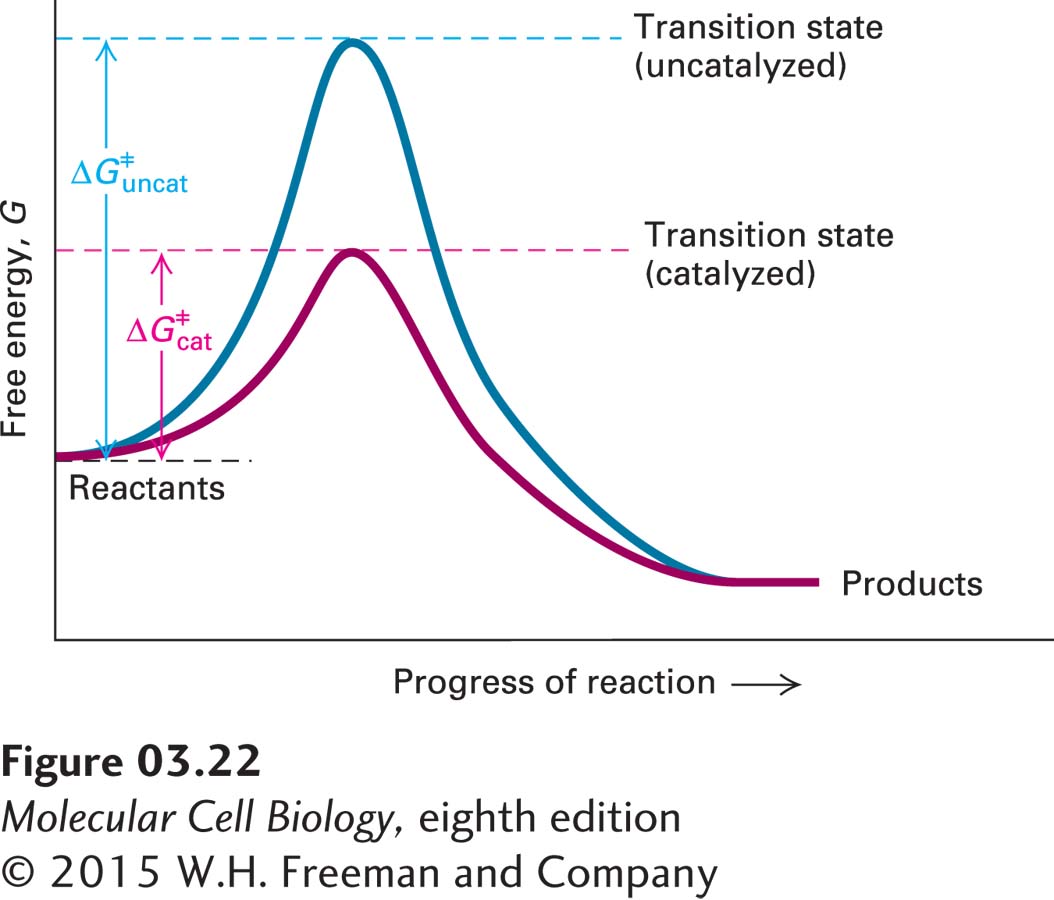
Like all catalysts (see Chapter 2), enzymes increase the rate of a reaction, but they do not affect the extent of a reaction, which is determined by the change in free energy (ΔG) between reactants and products, and they are not themselves permanently changed as a consequence of the reaction they catalyze. Enzymes increase the reaction rate by lowering the energy of the transition state, and therefore the activation energy required to reach it (Figure 3-22). In the test tube, catalysts such as charcoal and platinum facilitate reactions, but usually only at high temperatures or pressures, at extremes of high or low pH, or in organic solvents. Within cells, however, enzymes must function effectively in an aqueous environment at 37 °C and 1 atmosphere of pressure and at physiological pH values, usually 6.5–
91