In addition to exploiting the noncovalent regulators described above, cells can use covalent modifications to regulate the intrinsic activity of a protein. One of the most common covalent mechanisms for regulating protein activity is phosphorylation, the reversible addition of phosphate groups to hydroxyl groups on the side chains of serine, threonine, or tyrosine residues. Phosphorylated proteins are called phosphoproteins. Phosphorylation is catalyzed by enzymes called protein kinases, while the removal of phosphates, known as dephosphorylation, is catalyzed by phosphatases. The counteracting activities of kinases and phosphatases provide cells with a “switch” that can turn on or turn off the function of various proteins that are the substrates (or targets) of these enzymes (Figure 3-35). Sometimes phosphorylation sites are masked transiently by reversible covalent modification with the sugar N-acetylglucosamine (called O-GlcNAcylation), which is an additional means of covalent regulation. Phosphorylation changes a protein’s charge and to some extent its surface shape; it can also result in conformational changes. As a consequence, phosphorylation (or dephosphorylation) can influence the location of a protein within cells (e.g., its attachment to the inner surface of the plasma membrane), its intrinsic (e.g., enzymatic) activity, its ability to bind to other molecules, including metabolites, DNA, or other proteins, its ability to undergo further covalent modification, or its stability (rate of degradation). In addition, several conserved protein domains, such as the SH2 domain (see Figure 16-11), bind specifically to phosphorylated peptides. Thus phosphorylation can mediate the formation of protein complexes that can generate or extinguish a wide variety of cellular activities, discussed in many subsequent chapters.
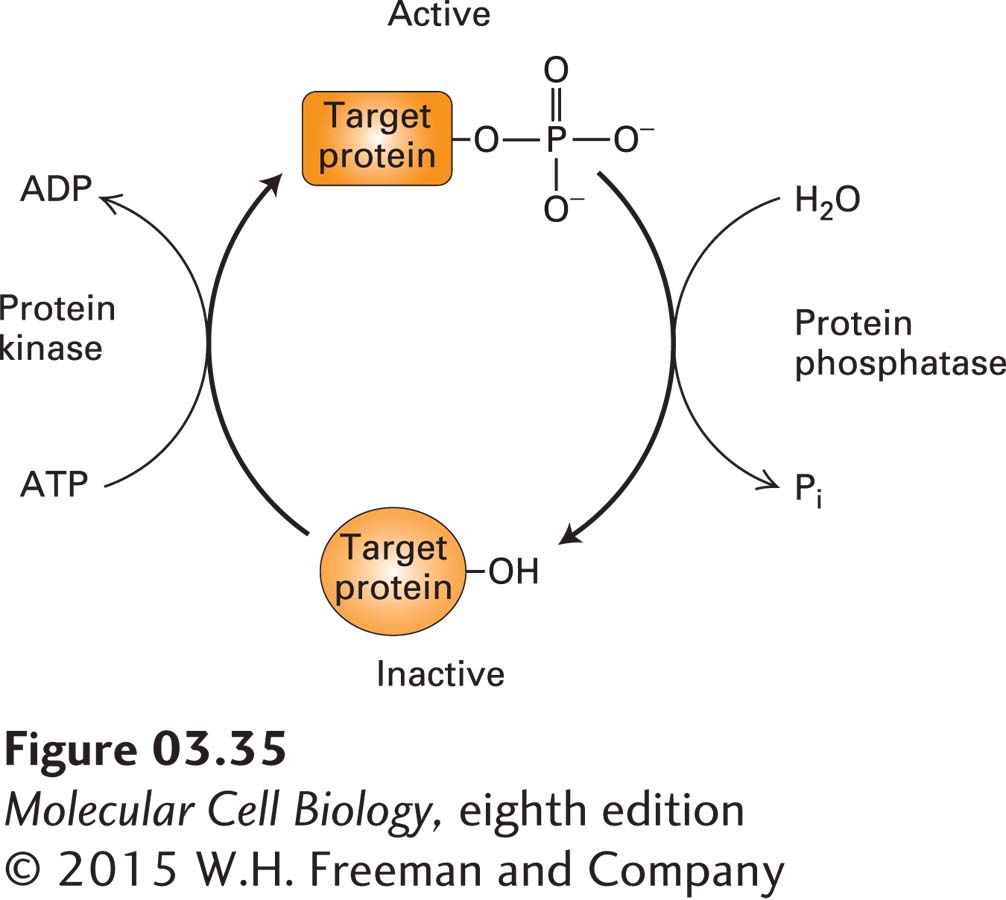
FIGURE 3-35 Regulation of protein activity by phosphorylation and dephosphorylation. The cyclic phosphorylation and dephosphorylation of a protein is a common cellular mechanism for regulating protein activity. In this example, the target protein is active (top) when phosphorylated and inactive (bottom) when dephosphorylated; some proteins have the opposite response to phosphorylation.
Nearly 3 percent of all yeast proteins are protein kinases or phosphatases, indicating the importance of phosphorylation and dephosphorylation reactions even in these simple cells. Analysis of the human genome indicates there are approximately 500 human protein kinases (the human “kinome”). All classes of proteins—including structural proteins, scaffolds, enzymes, membrane channels, and signaling molecules—have members regulated by kinase/phosphatase modifications. Different protein kinases and phosphatases are specific for different target proteins, often recognizing different linear sequences in which the residue to be phosphorylated is embedded, and so can regulate distinct cellular pathways, as discussed in later chapters. Some kinases have many targets, so that a single kinase can serve to integrate the activities of many targets simultaneously. Frequently, the target of a kinase or phosphatase is yet another kinase or phosphatase, creating a cascade effect. There are many examples of such kinase cascades, which permit amplification of a signal and many levels of fine-tuning (see Chapters 15 and 16).
