Centrifugation Can Separate Particles and Molecules That Differ in Mass or Density
The first step in a typical protein purification scheme is centrifugation. The principle behind centrifugation is that two types of particles in suspension (cells, cell fragments, organelles, or molecules) with different masses or densities will settle to the bottom of a test tube at different rates. Remember, mass is the weight of a sample (measured in daltons or molecular weight units), whereas density is the ratio of its mass to volume (often expressed as grams per liter because of the methods used to measure density). Proteins vary greatly in mass, but not in density. Unless a protein has an attached lipid or carbohydrate, its density will not vary by more than 15 percent from 1.37 g/cm3, the average protein density. Heavier or denser molecules settle, or sediment, more quickly than lighter or less dense molecules.
A centrifuge speeds sedimentation by subjecting particles in suspension to centrifugal forces as great as 1 million times the force of gravity, g, which can sediment particles as small as 10 kDa. Modern ultracentrifuges achieve these forces by reaching speeds of 150,000 revolutions per minute (rpm) or greater. However, small particles with masses of 5 kDa or less will not sediment uniformly even at such remarkably high rotation rates. The extraordinary technical achievements of modern ultracentrifuges can be appreciated by considering that they can rotate a several-pound rotor (about the size of an American football) that holds the samples in tubes at rates as high as 2500 revolutions per second!
Centrifugation is used for two basic purposes: (1) as a preparative technique to separate one type of material from others with the goal of obtaining enough of the material to perform subsequent experiments and (2) as an analytical technique to measure physical properties (e.g., molecular weight, density, shape, and equilibrium binding constants) of macromolecules. The sedimentation constant, s, of a protein is a measure of its sedimentation rate. The sedimentation constant is commonly expressed in Svedberg units (S), where a typical large protein complex is about 3–5S, a proteasome is 26S, and a eukaryotic ribosome is 80S.
Differential Centrifugation The most common initial step in protein purification from cells or tissues is the separation of water-soluble proteins from insoluble cellular material by differential centrifugation. A starting mixture, commonly a cell homogenate (mechanically broken cells), is poured into a tube and spun at a rotor speed, and for a period of time, that forces cell organelles such as nuclei as well as large unbroken cells or large cell fragments to collect as a pellet at the bottom; the soluble proteins remain in the supernatant (Figure 3-37a). The supernatant fraction then is poured off, and either it or the pellet can be subjected to other purification methods to separate the many different proteins that they contain.
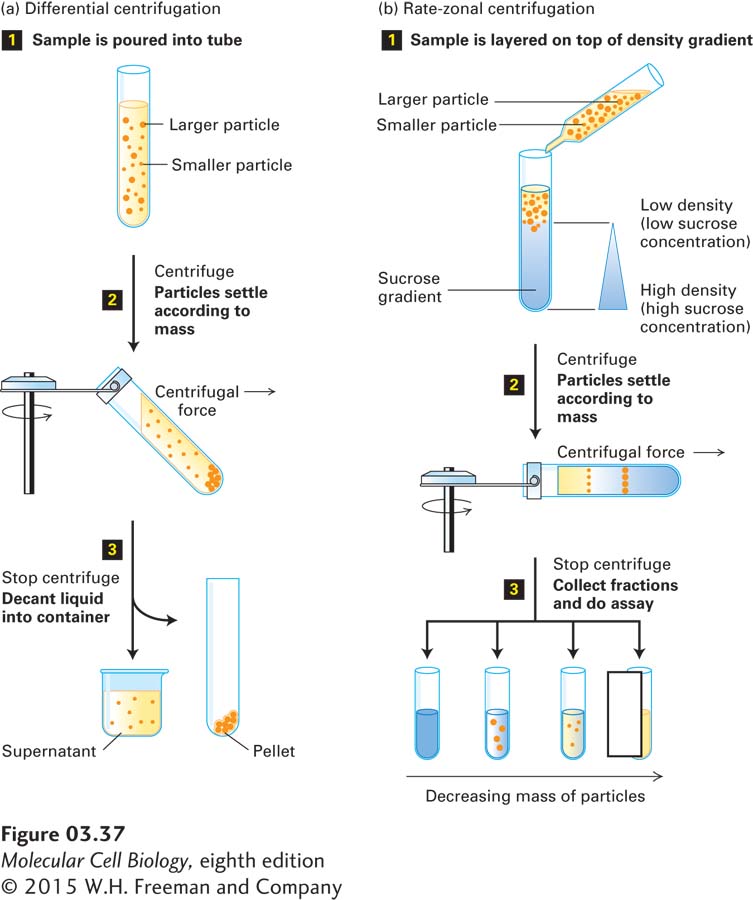
EXPERIMENTAL FIGURE 3-37 Centrifugation techniques separate particles that differ in mass or density. (a) In differential centrifugation, a cell homogenate or other mixture is spun long enough to sediment the larger particles (e.g., cell organelles, cells), which collect as a pellet at the bottom of the tube (step 2). The smaller particles (e.g., soluble proteins, nucleic acids) remain in the liquid supernatant, which can be transferred to another tube (step 3). (b) In rate-zonal centrifugation, a mixture is spun (step 1) just long enough to separate molecules that differ in mass but may be similar in shape and density (e.g., globular proteins, RNA molecules) into discrete zones within a density gradient commonly formed by a concentrated sucrose solution. Fractions are removed from the bottom of the tube and subjected to testing (assayed).
Rate-Zonal Centrifugation On the basis of differences in their masses, water-soluble proteins can be separated by centrifugation through a solution of increasing density, called a density gradient. A concentrated sucrose solution is commonly used to form a density gradient in a centrifuge tube (with higher concentrations of sucrose, and thus a higher solution density, toward the bottom of the tube, Figure 3-37b). When a protein mixture is placed on top of a sucrose density gradient in a tube and subjected to centrifugation, each protein in the mixture migrates down the tube at a rate controlled by the protein’s physical properties. All the proteins start from the thin layer of the sample that was placed at the top of the tube and separate into bands (actually, disks) of proteins of different masses as they travel at different rates through the gradient. In this separation technique, called rate-zonal centrifugation, samples are centrifuged just long enough to separate the molecules of interest into discrete bands, also called zones (see Figure 3-37b). If a sample is centrifuged for too short a time, the different protein molecules will not separate sufficiently. If a sample is centrifuged much longer than necessary, all the proteins will end up mixed together at the bottom of the tube.
Although the sedimentation rate is strongly influenced by particle mass, rate-zonal centrifugation is seldom effective in determining precise molecular weights because variations in shape also affect the sedimentation rate. The exact effects of shape are hard to assess, especially for proteins or other molecules, such as single-stranded nucleic acid molecules, that can assume many complex shapes. Nevertheless, rate-zonal centrifugation has proved to be a practical method for separating many different types of polymers and particles. A second density-gradient technique, called equilibrium density-gradient centrifugation, is used mainly to separate DNA, lipoproteins that carry lipids through the circulatory system, or organelles (see Figure 4-37).
