Secondary Structures Are the Core Elements of Protein Architecture
The second level in the hierarchy of protein structure is secondary structure. Secondary structures are stable spatial arrangements of segments of a polypeptide chain held together by hydrogen bonds between backbone amide and carbonyl groups and often involving repeating structural patterns. The propensity of a segment of a polypeptide chain to form any given secondary structure depends on its amino acid sequence (see Section 3.2). A single polypeptide may contain multiple types of secondary structure in various portions of the chain, depending on its sequence. The principal secondary structures are the alpha (α) helix, the beta (β) sheet, and the short U-shaped beta (β) turn. Parts of the polypeptide that don’t form these structures but nevertheless have a well-defined, stable shape are said to have an irregular structure. The term random coil applies to highly flexible parts of a polypeptide chain that have no fixed three-dimensional structure. In an average protein, 60 percent of the polypeptide chain exists as α helices and β sheets; the remainder of the molecule is in irregular structures, coils, and turns. Thus α helices and β sheets are the major internal supportive elements in most proteins. Here we explore the shapes of secondary structures and the forces that favor their formation. In later sections, we examine how arrays of secondary structure fold together into larger, more complex arrangements called tertiary structure.
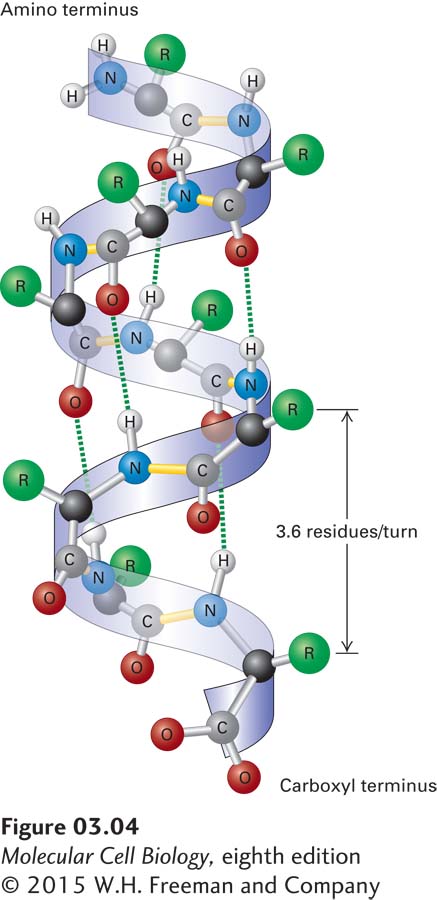
FIGURE 3-4 The α helix, a common secondary structure in proteins. The polypeptide backbone (seen as a ribbon) is folded into a spiral that is held in place by hydrogen bonds between backbone oxygen and hydrogen atoms. Only hydrogens involved in bonding are shown. The outer surface of the helix is covered by the side-chain R groups (green).
The α Helix In a polypeptide segment folded into an α helix, the backbone forms a spiral structure in which the carbonyl oxygen atom of each peptide bond is hydrogen-bonded to the amide hydrogen atom of the amino acid four residues farther along the chain in the direction of the C-terminus (Figure 3-4). Within an α helix, all the backbone amino and carboxyl groups are hydrogen-bonded to one another except at the very beginning and end of the helix. This periodic arrangement of bonds confers an amino-to-carboxy-terminal directionality on the helix because all the hydrogen bond acceptors (i.e., the carbonyl groups) have the same orientation (pointing in the downward direction in Figure 3-4), resulting in a structure in which there is a complete turn of the spiral every 3.6 residues. An α helix 36 amino acids long has 10 turns of the helix and is 5.4 nm long (0.54 nm per turn).
The stable arrangement of hydrogen-bonded amino acids in the α helix holds the backbone in a straight, rodlike cylinder from which the side chains point outward. The relative hydrophobic or hydrophilic quality of a particular helix within a protein is determined entirely by the characteristics of the side chains. In water-soluble proteins, hydrophilic helices with polar side chains extending outward tend to be found on the outside surfaces, where they can interact with the aqueous environment, whereas hydrophobic helices with nonpolar, hydrophobic side chains tend to be buried within the core of the folded protein. Proteins embedded in the hydrophobic core of cellular membranes (see Chapter 7) often use one or more hydrophobic helices that are 20–25 residues long to cross the membrane. The amino acid proline is usually not found in α helices because the covalent bonding of its amino group with a carbon in the side chain prevents its participation in stabilizing the backbone through normal hydrogen bonding. While the classic α helix is the most intrinsically stable and most common helical form in proteins, there are variations, such as more tightly or loosely twisted helices. For example, in a specialized helix called a coiled coil (described several sections farther on), the helix is more tightly wound (3.5 residues and 0.51 nm per turn).
The β Sheet Another type of secondary structure, the β sheet, consists of laterally packed β strands. Each β strand is a short (5–8-residue), nearly fully extended polypeptide segment. In contrast to the α helix, in which hydrogen bonds occur between the backbone amino and carboxyl groups of nearly adjacent residues, hydrogen bonds in the β sheet occur between backbone atoms in separate, but adjacent, β strands and are oriented perpendicularly to the chains of backbone atoms (Figure 3-5a). These distinct β strands (indicated as green and blue arrows in the figure) may be either within a single polypeptide chain, with short or long loops between the β strand segments, or on different polypeptide chains in a protein composed of multiple polypeptides. Figure 3-5b shows how two or more β strands align into adjacent rows, forming a nearly two-dimensional β pleated sheet (or simply pleated sheet), in which hydrogen bonds within the plane of the sheet hold the β strands together as the side chains stick out above and below the plane. Like α helices, β strands have a directionality defined by the orientation of the peptide bonds. Therefore, in a pleated sheet, adjacent β strands can be oriented in alternating opposite (antiparallel) directions (see Figure 3-5a) or in the same (parallel) direction (Figure 3-5c). In some proteins, β sheets form part of the hydrophobic core of the protein (described below) or the side of an open space that binds other molecules; in some proteins embedded in membranes, the β sheets curve around and form a hydrophilic central pore through which ions and small molecules may flow (see Chapter 7).
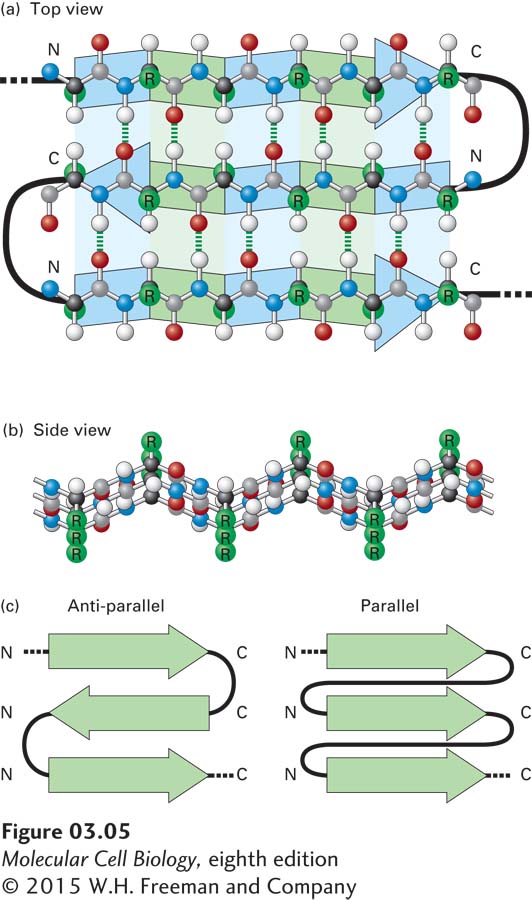
FIGURE 3-5 The β sheet, another common secondary structure in proteins. (a) Top view of a three-stranded β sheet. Each strand is highlighted by a ribbon-like arrow with alternating blue and green segments that is pointed with an N-to-C orientation, with the loops of connecting residues indicated by thick black lines. In this antiparallel β sheet, each strand (arrow) points in the direction opposite to that of the adjacent strand. The stabilizing hydrogen bonds between the β strands are indicated by green dashed lines. (b) Side view of an antiparallel β sheet. The projection of the R groups (green) above and below the plane of the sheet is obvious in this view. The fixed bond angles in the polypeptide backbone produce a pleated contour represented in panel (a) by the alternating colored segments. (c) Top view of two β sheets, whose individual strands (N-to-C orientations represented by arrows) are either antiparallel, in which the strands alternately point in opposite directions (left), or parallel, in which all strands point in the same direction (right).
The β Turn Composed of four residues, β turns are located on the surface of a protein, forming sharp bends that reverse the direction of the polypeptide backbone, often toward the protein’s interior. These short, U-shaped secondary structures are often stabilized by a hydrogen bond between their end residues (Figure 3-6). Glycine and proline are commonly found in β turns. The lack of a large side chain in glycine and the presence of a built-in bend in proline allow the polypeptide backbone to fold into a tight U shape. β Turns help long polypeptides fold into highly compact structures. A reversal in the direction of the polypeptide backbone may also be mediated by segments of the polypeptide that are longer than four residues and that form bends or loops. In contrast to tight β turns, which exhibit just a few well-defined conformations, longer loops can have many different conformations.
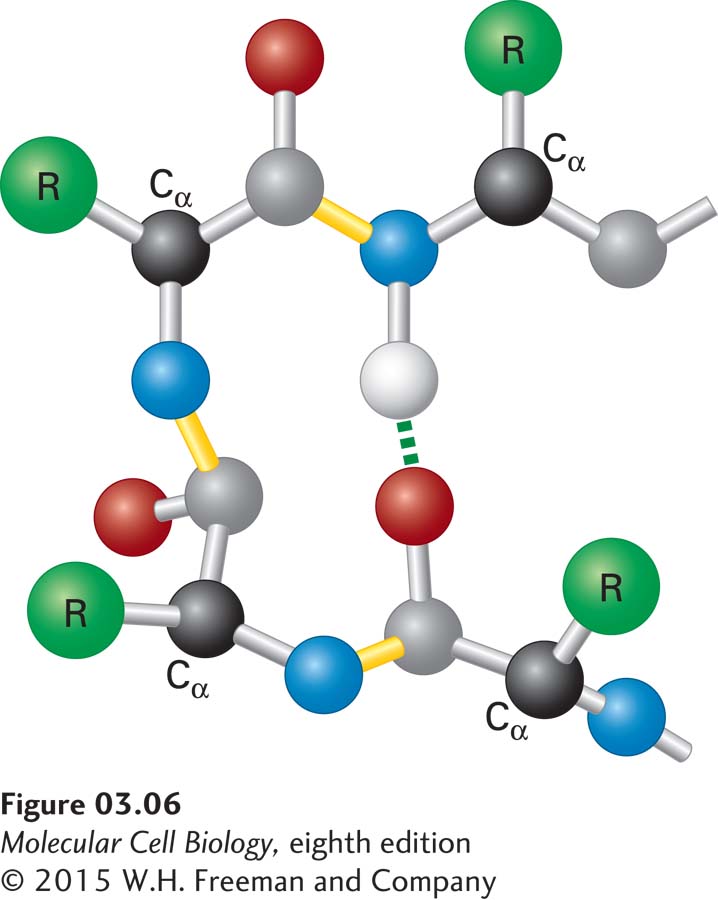
FIGURE 3-6 Structure of a β turn. Composed of four residues, β turns reverse the direction of a polypeptide chain (resulting in a 180° U-turn). The Cα carbons of the first and fourth residues are usually less than 0.7 nm apart, and those residues are often linked by a hydrogen bond. β turns facilitate the folding of long polypeptides into compact structures.


