Chapter Introduction
Culturing and Visualizing Cells
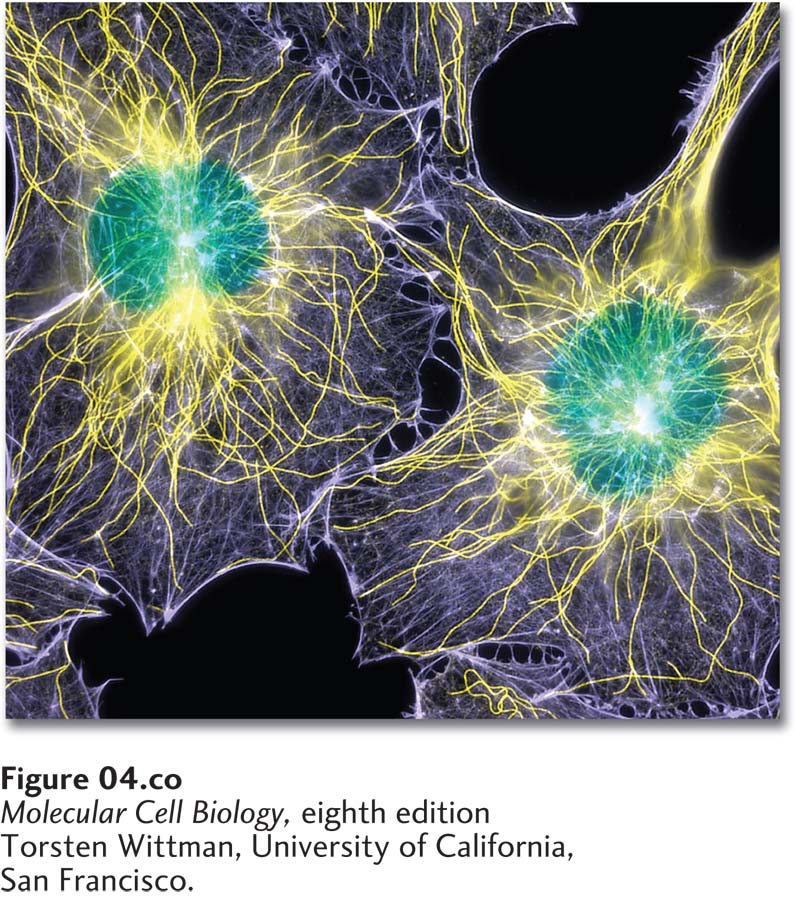
Fluorescence microscopy showing the locations of DNA (green), microtubules (yellow), and microfilaments (purple) in two cultured cells. The cells were chemically fixed and then rendered permeable to antibodies using a gentle detergent. Microtubules were stained with an antibody to tubulin; microfilaments were stained with a labeled toxin, phalloidin, that binds selectively to F-actin; and DNA was visualized with a DNA-binding dye.
[Torsten Wittman, University of California, San Francisco.]
OUTLINE
4.1 Growing and Studying Cells in Culture
4.2 Light Microscopy: Exploring Cell Structure and Visualizing Proteins Within Cells
4.3 Electron Microscopy: High-Resolution Imaging
4.4 Isolation of Cell Organelles
It is difficult to believe that just 200 years ago it was not yet appreciated that all living things are made of cells. In 1655, Robert Hooke used a primitive microscope to examine a piece of cork and saw an orderly arrangement of rectangles—the walls of the dead plant cells—that reminded him of monks’ cells in a monastery, so he coined the term cell. Shortly after this, Antoni van Leeuwenhoek’s observations of the microorganisms he saw in his simple microscope became the first description of live cells. Two hundred years later, Matthias Schleiden and Theodore Schwann proposed that cells constitute the fundamental unit of life in plants, animals, and single-celled organisms. Collectively, these discoveries were some of the greatest in biology and posed the question of how cells are organized and function.
Even today, many technical constraints hamper studies of cells in intact animals and plants. One alternative is the use of intact organs that are removed from animals and treated to maintain their physiological integrity and function. However, the organization of organs, even isolated ones, is sufficiently complex to pose numerous problems for research. Thus molecular cell biologists often conduct experimental studies on cells isolated from an organism. In Section 4.1, we learn how to maintain and grow diverse cell types and how to isolate specific types of cells from complex mixtures.
In many cases, isolated cells can be maintained in the laboratory under conditions that permit their survival and growth, a procedure known as culturing. Cultured cells have several advantages over intact organisms for cell biology research. Cells of a single specific type can be grown in culture, experimental conditions can be better controlled, and in many cases a single cell can be readily grown into a colony of many identical cells. The resulting strain of cells, which is genetically homogeneous, is called a clone. However, cultured cells are not in their native setting, so researchers are now growing and examining cells in three-dimensional environments to more closely mimic their situation in an animal.
Discoveries about cellular organization have been intimately tied to developments in microscopy. This is as true today as it was 400 years ago. Light microscopy initially revealed the beautiful internal organization of cells, and today highly sophisticated microscopes are continually being improved to probe deeper and deeper into the molecular mechanisms by which cells function. In Section 4.2, we discuss light microscopy and the long-standing but still valuable techniques that are available, and then examine several clever methods that have been developed more recently, culminating with the newest, cutting-edge technologies. A major advance came in the 1960s and 1970s with the development of immunofluorescence microscopy, which allows the localization of specific proteins within fixed cells, thus providing a static image of their location, as illustrated in the chapter-opening figure. Such studies led to the important understanding that the membranes and interior spaces of each type of organelle contain a distinctive group of proteins that are necessary for the organelle to carry out its unique functions. Another major advance came in the mid-1990s with the simple idea of expressing chimeric proteins—consisting of a protein of interest covalently linked to a naturally fluorescent protein—which enabled biologists to visualize the movements of individual proteins in live cells. Suddenly, the dynamic nature of cells could be appreciated, which changed the view of cells from the previously available static images. In addition, it presented a technological challenge: the more sensitive a microscope could be made to detect the fluorescent protein, the more information the investigator could glean from the data. It also opened up the development of fluorescent techniques to monitor protein-protein interactions in live cells, as well as a myriad of other sophisticated molecular technologies, some of which we also discuss in this section. For decades, light microscopy was constrained by the resolution of the light microscope—to about 200 nm—due to the limitations imposed by the wavelength of visible light. We discuss methods that have been developed over the last few years to “beat” this resolution barrier with the development of super-resolution microscopy.
Despite the amazing developments in light microscopy, visible light still provides too low a resolution to examine cells in fine ultrastructural detail. The electron microscope gives a much higher resolution, but the technology generally requires that the cell be fixed and sectioned, and therefore all cell movements are frozen in time. Electron microscopy also allows investigators to examine the structure of macromolecular complexes or single macromolecules. In Section 4.3, we outline the various approaches for preparing specimens for observation in the electron microscope and describe the types of information that can be derived from them.
Light and electron microscopy revealed that all eukaryotic cells—whether of fungal, plant, or animal origin—contain a similar repertoire of membrane-limited compartments termed organelles. In parallel with the developments in microscopy, subcellular fractionation methods were developed that have enabled cell biologists to isolate individual organelles to a high degree of purity. These techniques, detailed in Section 4.4, continue to provide important information about the protein composition and biochemical function of organelles.
