The Resolution of the Conventional Light Microscope Is About 0.2 µm
All microscopes produce a magnified image of a small object, but the nature of the image depends on the type of microscope employed and on the way the specimen is prepared. The compound microscope, used in conventional bright-field light microscopy, contains several lenses that magnify the image of a specimen under study (Figure 4-9a, b). The total magnification is a product of the magnification of the individual lenses: if the objective lens, the lens closest to the specimen, magnifies 100-fold (a 100× lens, the maximum usually employed), and the projection lens that focuses the image on a camera magnifies 10-fold, the final magnification will be 1000-fold. Alternatively, if the light is directed to an ocular or eyepiece lens that magnifies 10-fold, the final magnification recorded by the human eye will also be 1000-fold.
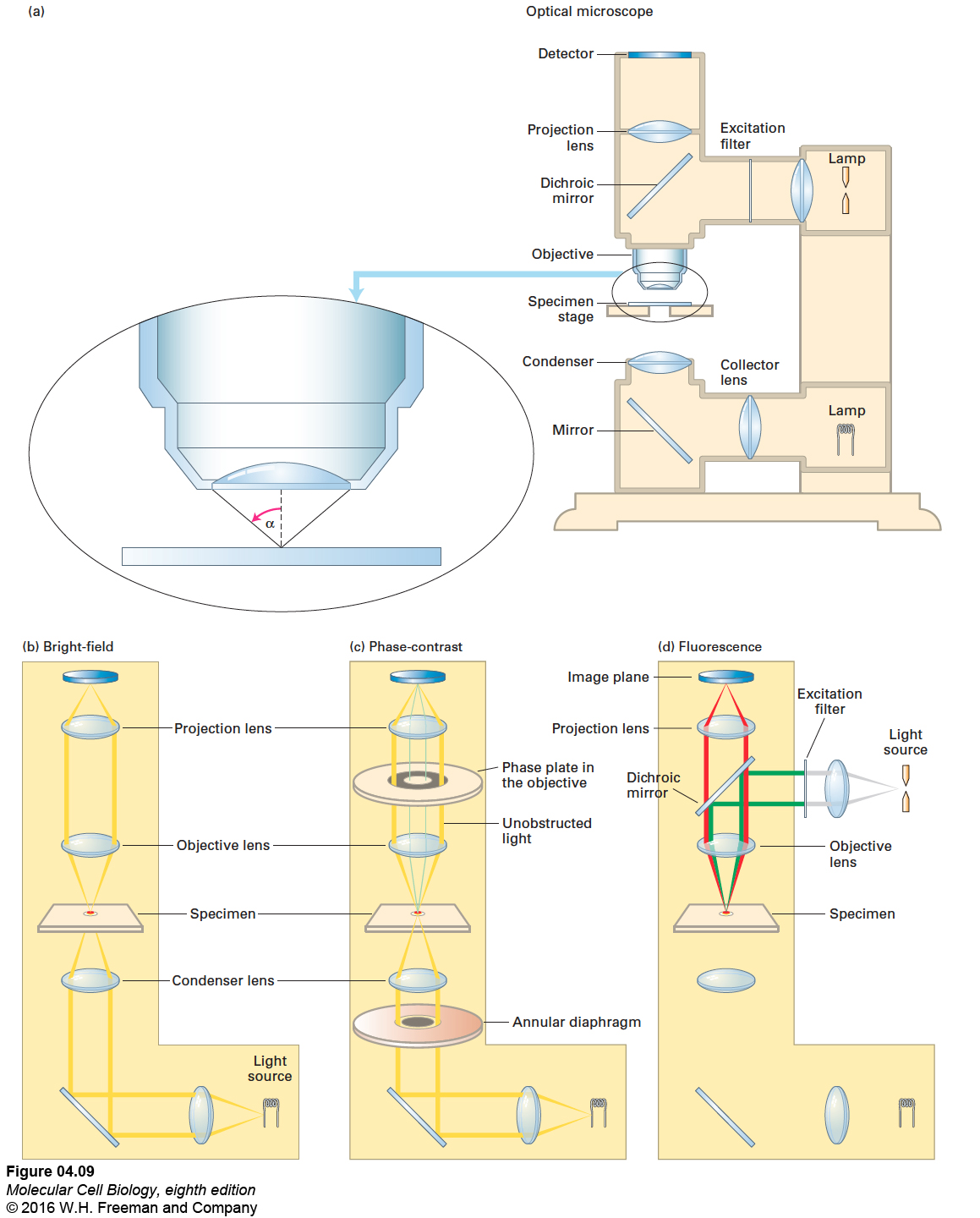
FIGURE 4-9 Optical microscopes are commonly configured for bright-field, phase-contrast, or fluorescence microscopy. (a) In a typical light microscope, the specimen is usually mounted on a transparent glass slide and positioned on the movable specimen stage. (b) In bright-field light microscopy, light from a tungsten lamp is focused on the specimen by a condenser lens below the stage; the light travels the pathway shown in yellow. (c) In phase-contrast microscopy, incident light passes through an annular diaphragm, which focuses a circular annulus (ring) of light on the specimen. Light that passes unobstructed through the specimen is focused by the objective lens onto the thicker gray ring of the phase plate, which absorbs some of the direct light and alters its phase by one-quarter of a wavelength. If a specimen refracts (bends) or diffracts the light, the phase of some light waves is altered (green lines), and the light waves pass through the clear region of the phase plate. The refracted and unrefracted light is recombined at the image plane to form the image. (d) In fluorescence microscopy, a beam of light from a mercury lamp (gray lines) is directed to the excitation filter, which allows only the correct wavelength of light to pass through (green lines). The light is then reflected off a dichroic mirror and through the objective lens, which focuses it on the specimen. The fluorescent light emitted by the specimen (red lines) passes up through the objective lens, then through the dichroic mirror, and is focused and recorded on the detector at the image plane.
The most important property of any microscope, however, is not its magnification, but its resolving power, or resolution: the ability to distinguish between two very closely positioned objects. Merely enlarging the image of a specimen accomplishes nothing if the image is blurred. The resolution of a microscope lens is numerically equivalent to D, the minimum distance between two distinguishable objects. The smaller the value of D, the better the resolution. The value of D is given by the equation

where α is the angular aperture, or half-angle, of the cone of light entering the objective lens from the specimen (Figure 4-9a), N is the refractive index of the medium between the specimen and the objective lens (i.e., the relative velocity of light in the medium compared with the velocity in air), and λ is the wavelength of the incident light. Resolution is improved by using shorter wavelengths of light (decreasing the value of λ) or by gathering more light (increasing either N or α). Lenses for high-resolution microscopy are designed to work with oil between the lens and the specimen since oil has a higher refractive index (1.56, compared with 1.0 for air and 1.3 for water). To maximize the angle α, and hence sin α, the lenses are also designed to focus very close to the thin coverslip covering the specimen. The term N sin α is known as the numerical aperture (NA) and is usually marked on the objective lens. A good high-magnification lens has an NA of about 1.4, and the very best lenses—which cost as much as a medium-sized car—have a value approaching 1.5. Notice that the magnification is not part of this equation.
Owing to limitations in the values of α, λ, and N based on the physical properties of light, the limit of resolution of a light microscope using visible light is about 0.2 µm (200 nm). No matter how many times the image is magnified, a conventional light microscope can never resolve objects that are closer than about 0.2 µm apart or reveal details smaller than about 0.2 µm in size. However, some new and sophisticated technologies devised to “beat” this resolution barrier can resolve objects just a few nanometers apart; we discuss such super-resolution microscopes in a later section.
Despite its lack of resolution, a conventional microscope can track a single object to within a few nanometers. If we know the precise size and shape of an object—say, a 5-nm sphere of gold that is attached to an antibody that is in turn bound to a cell-surface protein on a live cell—and if we use a camera to rapidly take multiple digital images, then a computer can calculate the average position to reveal the center of the object to within a few nanometers. In this way, computer algorithms can be used to track a single object—in this case, the location and movement with time of a cell-surface protein labeled with the gold-tagged antibody—more precisely than would be possible based on the light microscope’s resolution alone. This technique has been used to measure nanometer-sized steps as molecules and vesicles move along cytoskeletal filaments (see Figures 17-28 and 17-29).
