Phase-Contrast and Differential-Interference-Contrast Microscopy Visualize Unstained Live Cells
Cells are about 70 percent water, 15 percent protein, 6 percent RNA, and contain smaller amounts of lipids, DNA, and small molecules. Since none of these major classes of molecules are colored, and since they hardly impede the transmission of light, special methods must be used to see cells in a microscope. For example, the simplest microscopes view cells under bright-field optics (Figure 4-9b), and little detail can be seen (Figure 4-10). Two common methods for imaging live cells and unstained tissues to generate contrast take advantage of differences in the refractive index and thickness of cellular materials. These methods, called phase-contrast microscopy and differential-interference-contrast (DIC) microscopy (or Nomarski interference microscopy), produce images that differ in appearance and reveal different features of cell architecture. Figure 4-10 compares images of live, cultured cells obtained with these two methods and with standard bright-field microscopy. Since optical microscopes are expensive, they are often set up to perform many different types of microscopy on the same microscope stand (see Figure 4-9a–d).
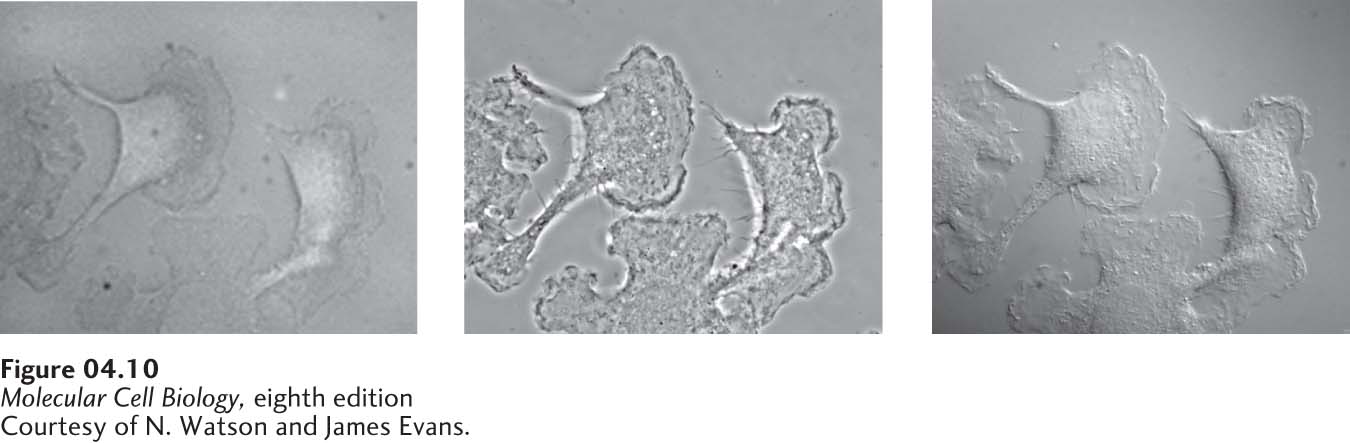
FIGURE 4-10 Live cells can be visualized by microscopy techniques that generate contrast by interference. These micrographs show live, cultured macrophage cells viewed by bright-field microscopy (left), phase-contrast microscopy (middle), and differential-interference-contrast (DIC) microscopy (right). In a phase-contrast image, cells are surrounded by alternating dark and light bands; in-focus and out-of-focus details are simultaneously imaged in a phase-contrast microscope. In a DIC image, cells appear in pseudorelief. Because only a narrow in-focus region is imaged, a DIC image is an optical slice through the object.
[Courtesy of N. Watson and James Evans.]
Phase-contrast microscopy generates an image in which the degree of darkness or brightness of a region of a specimen depends on the refractive index of that region. Light moves more slowly in a medium with a higher refractive index. Thus a beam of light is refracted (bent) once as it passes from the medium into a transparent object and again when it departs. In a phase-contrast microscope, a cone of light generated by an annular diaphragm in the condenser lens illuminates the specimen (see Figure 4-9c). The light passes through the specimen into the objective lens, and the unobstructed direct light passes through a region of the phase plate that both transmits only a small percentage of the light and changes its phase slightly. The part of a light wave that passes through a specimen will be refracted and will be out of phase (out of synchrony) with the part of the wave that does not pass through the specimen. How much their phases differ depends on the difference in refractive index along the two paths and on the thickness of the specimen. The refracted and unrefracted light is recombined at the image plane to form the image. If the two parts of the light wave are recombined, the resultant light will be brighter if they are in phase and less bright if they are out of phase. Phase-contrast microscopy is suitable for observing single cells or thin cell layers, but not thick tissues. It is particularly useful for examining the location and movement of larger organelles in live cells.
DIC microscopy, which is based on splitting the light into two perpendicular components before passing them through the specimen and then recombining them to observe their interference pattern, is the method of choice for visualizing extremely small details and thick objects. Contrast is generated by differences in the refractive index of the object and of its surrounding medium. In DIC images, objects appear to cast a shadow to one side. The “shadow” primarily represents a difference in the refractive index of a specimen rather than its topography. DIC microscopy easily defines the outlines of large organelles, such as the nucleus and vacuole. In addition to having a “relief”-like appearance, a DIC image is a thin optical section, or slice, through the object (Figure 4-10, right). Thus details of the nucleus in thick specimens (e.g., an intact Caenorhabditis elegans roundworm; see Figure 21-25d) can be observed in a series of such optical sections, and the three-dimensional structure of the object can be reconstructed by combining the individual DIC images.
Both phase-contrast and DIC microscopy can be used in time-lapse microscopy, in which the same cell is photographed at regular intervals over time to generate a movie. This procedure allows the observer to study cell movement, provided the microscope’s stage can control the temperature of the specimen and the appropriate environment.
