Imaging Subcellular Details Often Requires That Specimens Be Fixed, Sectioned, and Stained
As we have seen, live cells and tissues generally do not absorb light, so they are nearly invisible in a light microscope. Although cells can be visualized by the special techniques we have just discussed, these methods do not reveal the fine details of structure.
Specimens for light microscopy are commonly fixed with a solution containing chemicals that cross-
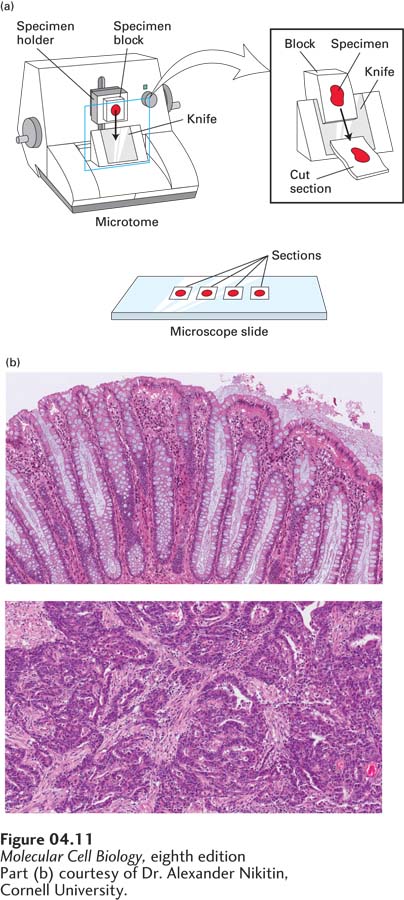
A final step in preparing a specimen for light microscopy is to stain it so as to visualize the main structural features of the cell or tissue. Many chemical stains bind to molecules that have specific features. For example, histological samples are often stained with hematoxylin and eosin (“H&E stain”). Hematoxylin binds to basic amino acids (lysine and arginine) on many different kinds of proteins, whereas eosin binds to acidic molecules (such as DNA and side chains of the amino acids aspartate and glutamate). Because of their different binding properties, these dyes stain various cell types sufficiently differently that they are distinguishable visually (Figure 4-11b). If an enzyme catalyzes a reaction that produces a colored or otherwise visible precipitate from a colorless precursor, that enzyme can be detected in cell sections by their colored reaction products. Such staining techniques, although once quite common, have been largely replaced by other techniques for visualizing particular proteins, as we discuss next.
143