Live cell fluorescence imaging reveals the locations and bulk dynamics of populations of fluorescent molecules, but it doesn’t tell you how dynamic individual molecules are. For example, if we see that a GFP-labeled protein forms a patch at the surface of a cell, does this represent a stable collection of fluorescent protein molecules or a dynamic equilibrium, with fluorescent proteins coming in and out of the patch? We can investigate this question by observing the dynamics of the molecules in the patch (Figure 4-23). If we use a high-intensity light to permanently bleach the fluorochrome (e.g., GFP) in the patch, there will initially be no fluorescence coming from it, and it will look dark in the fluorescence microscope. However, if the components in the patch are in dynamic equilibrium with unbleached molecules elsewhere in the cell, the bleached molecules will be replaced by unbleached ones, and the fluorescence will begin to come back. The rate of fluorescence recovery is a measure of the dynamics of the molecules. This technique, known as fluorescence recovery after photobleaching (FRAP), has revealed how very dynamic many components of cells are. For example, it has been used to determine the diffusion coefficient of membrane proteins (see Figure 7-10) and the dynamics of specific components of the secretory pathway. In another approach to measuring the dynamics of tagged proteins, variants of fluorescent proteins have been developed that can be switched, using a laser of an appropriate wavelength, from emitting green light to emitting red light. In this way, the dynamics of the switched population of red-emitting molecules can be imaged in live cells.
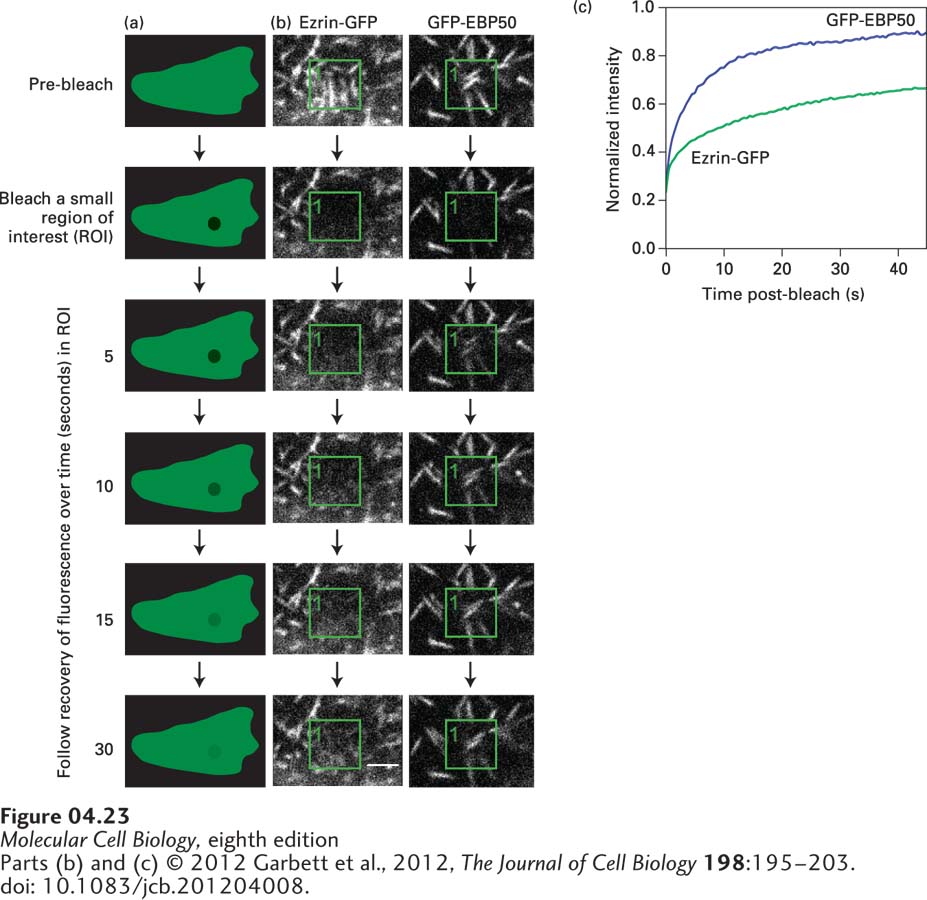
EXPERIMENTAL FIGURE 4-23 Fluorescence recovery after photobleaching (FRAP) reveals the dynamics of molecules. In a live cell, following the distribution of a GFP-labeled protein provides a view of the overall distribution of the protein, but it doesn’t tell us how dynamic populations of individual molecules might be. (a) In FRAP, the GFP fluorochrome is bleached by a short burst of strong laser light focused on the region of interest (ROI). The light rapidly bleaches the molecules irreversibly, so they are not detected again. Restoration of fluorescence in the region tells us that unbleached molecules have moved into the ROI. (b) FRAP was used to determine the exchange rate of EBP50 and ezrin, two components of microvilli (seen as white lines), on the apical surface of epithelial cells. In cells expressing either GFP-EBP50 or ezrin-GFP, the GFP in a small region indicated by the green box was bleached, and recovery by exchange with unbleached protein was followed. The fluorescence of GFP-EBP50 returns very fast, indicating it has a fast exchange rate, whereas the fluorescence of ezrin-GFP returns slowly, indicating a slower exchange rate. (c) By quantifying the recovery, the dynamic properties of EBP50 and ezrin can be established.
[Parts (b) and (c) © 2012 Garbett et al., 2012, The Journal of Cell Biology 198:195–203. doi: 10.1083/jcb.201204008.]
