Classic Experiment 4-1: Separating Organelles
H. Beaufay et al., 1964, Biochem. J. 92:191
In the 1950s and 1960s, scientists used two techniques to study cell organelles: microscopy and fractionation. Christian de Duve was at the forefront of cell fractionation. In the early 1950s, he used centrifugation to distinguish a new organelle, the lysosome, from previously characterized fractions: the nucleus, the mitochondrial-rich fraction, and the microsomes. Soon thereafter, he used equilibrium density-gradient centrifugation to uncover yet another organelle.
Eukaryotic cells are highly organized and composed of cell structures known as organelles that perform specific functions. Although microscopy has allowed biologists to describe the location and appearance of various organelles, it is of limited use in uncovering an organelle’s function. To do this, cell biologists have relied on a technique known as cell fractionation, in which cells are broken open and the cellular components are separated on the basis of size, mass, and density using a variety of centrifugation techniques. Scientists can then isolate and analyze cell components of different densities, called fractions. Using this method, biologists had divided the cell into four fractions: nuclei, mitochondrial-rich fraction, microsomes, and cell sap.
De Duve was a biochemist interested in the subcellular locations of metabolic enzymes. He had already completed a large body of work on the fractionation of liver cells, in which he had determined the subcellular locations of numerous enzymes. By locating these enzymes in specific cell fractions, he could begin to elucidate the functions of organelles. He noted that his work was guided by two hypotheses: the “postulate of biochemical homogeneity” and “the postulate of single location.” In short, these hypotheses propose that the entire composition of a subcellular population will contain the same enzymes, and that each enzyme is located at a discrete site within the cell. Armed with these hypotheses and the powerful tool of centrifugation, de Duve further subdivided the mitochondrial-rich fraction. First, he identified the light mitochondrial fraction, which is made up of hydrolytic enzymes that are now known to be components of the lysosome. Then, in a series of experiments described here, he identified another discrete subcellular fraction, which he called the peroxisome, within the mitochondrial-rich fraction.
De Duve studied the distribution of enzymes in rat liver cells. Highly active in energy metabolism, the liver contains a number of useful enzymes to study. To look for the presence of various enzymes during the fractionation, de Duve relied on known tests, called enzyme assays, for enzyme activity. To retain maximum enzyme activity, he had to take precautions, which included performing all fractionation steps at 0 °C to reduce protease activity.
De Duve used rate-zonal centrifugation to separate cellular components by successive centrifugation steps. He removed a rat’s liver and broke it apart by homogenization. The crude preparation of homogenized cells was then subjected to relatively low-speed centrifugation. This initial step separated the cell nucleus, which collects as sediment at the bottom of the tube, from the cytoplasmic extract, which remains in the supernatant. Next de Duve further subdivided the cytoplasmic extract into heavy mitochondrial fraction, light mitochondrial fraction, and microsomal fraction. He separated the cytoplasm by employing successive centrifugation steps of increasing force. At each step he collected and stored the fractions for subsequent enzyme analysis. Once the fractionation was complete, de Duve performed enzyme assays to determine the subcellular distribution of each enzyme. He then graphically plotted the distribution of the enzyme throughout the cell. As had been shown previously, the activity of cytochrome oxidase, an important enzyme in the electron-transfer system, was found primarily in the heavy mitochondrial fractions. The microsomal fraction was shown to contain another previously characterized enzyme, glucose-6-phosphatase. The light mitochondrial fraction, which is made up of lysosomes, showed the characteristic acid phosphatase activity. Unexpectedly, de Duve observed a fourth pattern when he assayed uricase activity. Rather than following the pattern of the reference enzymes, uricase activity was sharply concentrated within the light mitochondrial fraction. This sharp concentration, in contrast to the broad distribution he expected, suggested to de Duve that the uricase might be secluded in another subcellular population separate from the lysosomal enzymes.
To test this theory, de Duve employed a technique known as equilibrium density-gradient centrifugation, which separates macromolecules on the basis of density. Equilibrium density-gradient centrifugation can be performed using a number of different gradients, including sucrose and glycogen. In addition, the gradient can be made up in either water or “heavy water,” which contains the hydrogen isotope deuterium in place of hydrogen. In his experiment, de Duve separated the mitochondrial-rich fraction prepared by rate-zonal centrifugation in each of these different gradients (see Figure 4-37). If uricase were part of a separate subcellular compartment, it would separate from the lysosomal enzymes in each gradient tested. De Duve performed the fractionations in this series of gradients, then performed enzyme assays as before. In each case, he found uricase in a separate population than the lysosomal enzyme acid phosphatase and the mitochondrial enzyme cytochrome oxidase (Figure 1). By repeatedly observing uricase activity in a distinct fraction from the activity of the lysosomal and mitochondrial enzymes, de Duve concluded that uricase was part of a separate organelle. The experiment also showed that two other enzymes, catalase and D–amino acid oxidase, segregated into the same fractions as uricase. Because each of these enzymes either produced or used hydrogen peroxide, de Duve proposed that this fraction represented an organelle responsible for peroxide metabolism and dubbed it the peroxisome.
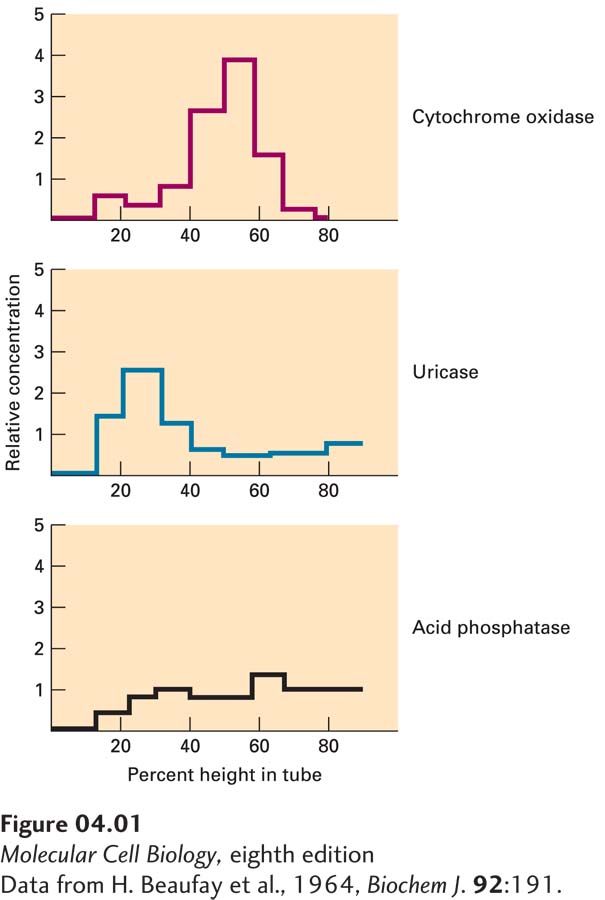
FIGURE 1 Graphic representation of the enzyme analysis of products from a sucrose gradient. The mitochondrial-rich fraction was separated as depicted in Figure 4-37, and then enzyme assays were performed. The relative concentration of active enzyme is plotted on the y axis; the height in the tube is plotted on the x axis. The peak activities of cytochrome oxidase (top) and acid phosphatase (bottom) are observed near the top of the tube. The peak activity of uricase (middle) migrates to the bottom of the tube.
[Data from H. Beaufay et al., 1964, Biochem J. 92:191.]
De Duve’s work on cellular fractionation provided an insight into the function of cell structures as he sought to map the locations of known enzymes. Examining the inventory of enzymes in a given cell fraction gave him clues to its function. His careful work resulted in the uncovering of two organelles: the lysosome and the peroxisome. His work also provided important clues to the organelles’ function. The lysosome, where de Duve found so many potentially destructive enzymes, is now known to be an important site for degradation of biomolecules. The peroxisome has been shown to be the site of fatty acid and amino acid oxidation, reactions that produce a large amount of hydrogen peroxide. In 1974, de Duve received the Nobel Prize in Physiology or Medicine in recognition of his pioneering work.
