Flow Cytometry Separates Different Cell Types
Some cell types differ sufficiently in density that they can be separated on the basis of this physical property. White blood cells (leukocytes) and red blood cells (erythrocytes), for instance, have very different densities because erythrocytes have no nucleus; thus these cells can be separated by equilibrium density-gradient centrifugation (described in Section 4.4). Most cell types cannot be differentiated so easily, so other techniques, such as flow cytometry, must be used to separate them.
To separate one type of cell from a complex mixture, it is necessary to have some way to mark and then sort out the desired cells. As we will see below, it is possible to mark cells by expressing a fluorescent protein in them, but if only a few cells in the population express the protein, how can we sort them from the nonfluorescent ones? The cells can be analyzed in a flow cytometer. This machine flows cells past a laser beam that measures the light that they scatter and the fluorescence that they emit; thus it can quantify the cells expressing the fluorescent protein in a mixture. A fluorescence-activated cell sorter (FACS), which is based on flow cytometry, can both analyze the cells and select the few fluorescent cells from thousands of others and sort them into a separate culture dish (Figure 4-2). To achieve this, the cells are mixed with a buffer and forced through a vibrating nozzle to generate tiny droplets. The concentration of cells is adjusted so that most of the droplets do not contain cells, and the ones that do contain only one. Just before the nozzle, the stream of cells passes through a laser beam so that the presence and size of a cell can be recorded from the scattered light using one detector, and the amount of fluorescent light emitted can be quantified using a second, fluorescent light detector. If a cell is present in a droplet, the droplet is given a negative electric charge as it emerges from the nozzle. The stream of droplets then passes through two plates that generate an electric field proportional to the fluorescence detected from the cell in the droplet. This field generates a force that moves charged droplets out of the stream of uncharged droplets and into a collection tube. Since the amount of force applied is proportional to the fluorescence emitted by the cell in the droplet, cells with different levels of fluorescence can be collected. Having been sorted from other cells, the selected cells can be grown in culture.
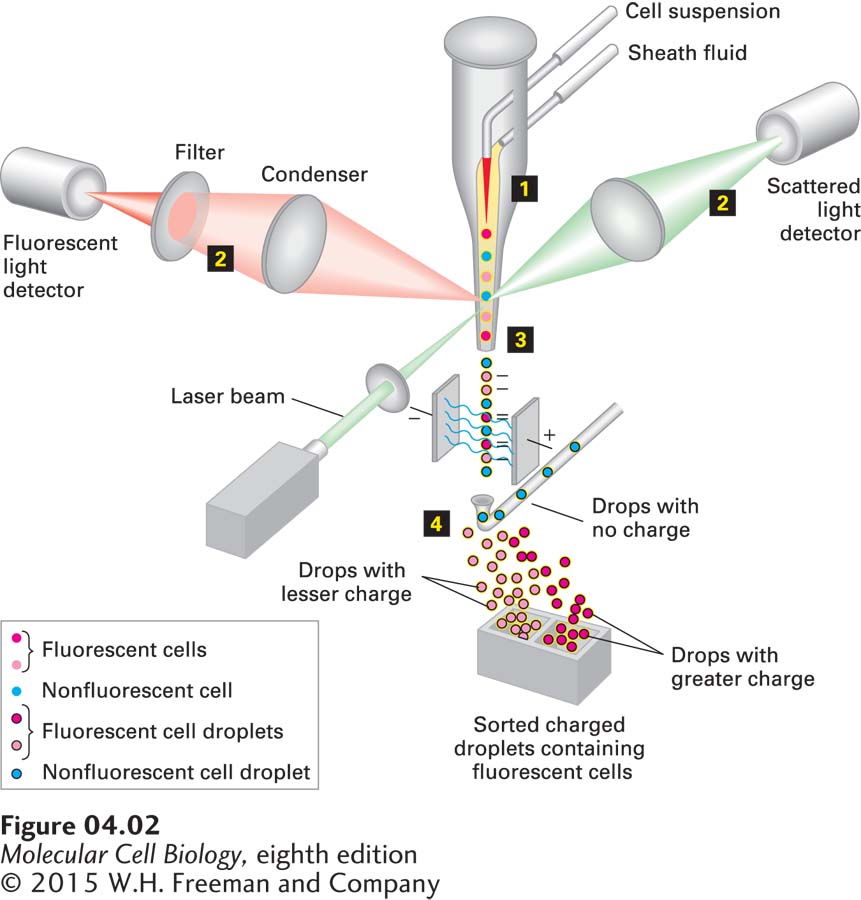
FIGURE 4-2 A fluorescence-activated cell sorter (FACS) separates cells having different levels of fluorescence. Step 1: A concentrated suspension of labeled cells is mixed with a buffer (the sheath fluid) so that the cells pass single file through a laser light beam. Step 2: Both the fluorescent light emitted and the light scattered by each cell are measured; from measurements of the scattered light, the size and shape of the cell can be determined. Step 3: The suspension is then forced through a nozzle, which forms tiny droplets containing at most a single cell. At the time of formation at the nozzle tip, each droplet containing a cell is given a negative electric charge proportional to the fluorescence of that cell determined from the earlier measurement. Step 4: Droplets now pass through an electric field, so that those with no charge are discarded, whereas those with different electric charges are separated and collected. Because it takes only milliseconds to sort each droplet, as many as 10 million cells per hour can pass through the machine.
The FACS procedure is commonly used to purify the different types of white blood cells, each of which bears on its surface one or more distinctive proteins and so will bind monoclonal antibodies specific for its proteins. If a cell mixture is incubated with a fluorescent dye linked to the antibody to a specific cell-surface protein, only the desired cells will be fluorescent. Only the T cells of the immune system, for instance, have both CD3 and Thy1.2 proteins on their surfaces. The presence of these surface proteins allows T cells to be separated easily from other types of blood cells or spleen cells (Figure 4-3).
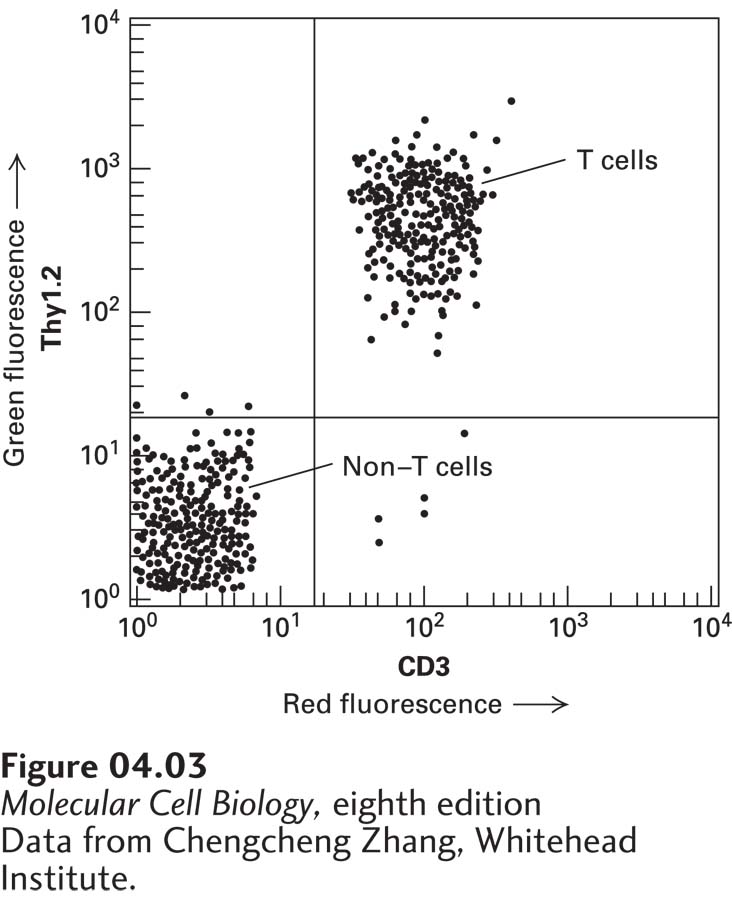
EXPERIMENTAL FIGURE 4-3 T cells bound to fluorescence-tagged antibodies to two cell-surface proteins are separated from other white blood cells by FACS. Spleen cells from a mouse were treated with a red fluorescent monoclonal antibody specific for the CD3 cell-surface protein and with a green fluorescent monoclonal antibody specific for a second cell-surface protein, Thy1.2. As the cells were passed through a FACS, the intensity of the green and red fluorescence emitted by each cell was recorded. Each dot represents a single cell. This plot of green fluorescence (vertical axis) versus red fluorescence (horizontal axis) for thousands of spleen cells shows that about half of them—the T cells—express both CD3 and Thy1.2 proteins on their surfaces (upper-right quadrant). The remaining cells, which exhibit low fluorescence (lower-left quadrant), express only background levels of these proteins and are other types of white blood cells. Note the logarithmic scale on both axes.
[Data from Chengcheng Zhang, Whitehead Institute.]
Other uses of flow cytometry include the measurement of a cell’s DNA and RNA content and the determination of its general shape and size. The FACS can make simultaneous measurements of the size of a cell (from the amount of scattered light) and the amount of DNA that it contains (from the amount of fluorescence emitted from a DNA-binding dye). Measurements of the DNA content of individual cells are used to follow replication of DNA as the cells progress through the cell cycle (see Chapter 19).
An alternative method for separating specific types of cells uses small magnetic beads coupled to antibodies to a specific cell-surface molecule. For example, to isolate T cells, the beads are coated with a monoclonal antibody specific for a surface protein such as CD3 or Thy1.2. Only cells with these proteins will stick to the beads, which can be recovered from the preparation by adhesion to a small magnet on the side of a test tube.

