Base Excision Repairs T-G Mismatches and Damaged Bases
In humans, the most common type of point mutation is a change from a C to a T, which is caused by deamination of 5-methyl C (see Figure 5-34). The conceptual problem with base excision repair in this case is determining which is the normal and which is the mutant DNA strand. But since a G·T mismatch is almost invariably caused by chemical conversion of C to U or 5-methyl C to T, the repair system evolved to remove the T and replace it with a C.
The G·T mismatch is recognized by a DNA glycosylase that flips the thymine base out of the helix and then hydrolyzes the bond that connects it to the sugar-phosphate DNA backbone. Following this initial incision, an endonuclease, APE1, cuts the DNA strand near the now abasic site. The deoxyribose phosphate lacking the base is then removed and replaced with a C by a specialized DNA polymerase that reads the G in the template strand (Figure 5-35).
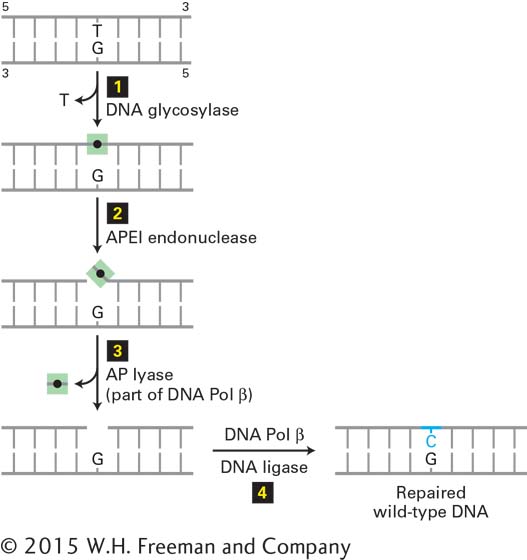
FIGURE 5-35 Base excision repair of a T·G mismatch. A DNA glycosylase specific for G·T mismatches, which are usually formed by deamination of 5-methyl C (see Figure 5-34), flips the thymine base out of the helix and then cuts it away from the sugar-phosphate DNA backbone (step 1), leaving just the deoxyribose phosphate (black dot). An endonuclease specific for the resultant abasic site (apurinic endonuclease I, APE1) then cuts the DNA backbone (step 2), and the deoxyribose phosphate is removed by an endonuclease, apurinic lyase (AP lyase) associated with DNA polymerase β, a specialized DNA polymerase used in repair (step 3). The gap is then filled in by DNA Pol β and sealed by DNA ligase (step 4), restoring the original G·C base pair. See O. Schärer, 2003, Angewandte Chemie 42:2946.
As mentioned earlier, this repair must take place prior to DNA replication because the incorrect base in this pair, T, occurs naturally in normal DNA. Consequently, it would be able to engage in normal Watson-Crick base pairing during replication, generating a stable point mutation that would no longer be recognized by repair mechanisms (see Figure 5-34, step 2).
Human cells contain a battery of glycosylases, each of which is specific for a different set of chemically modified DNA bases. For example, one glycosylase removes 8-oxyguanine, an oxidized form of guanine, allowing its replacement by an undamaged G, and others remove bases modified by alkylating agents. The resulting abasic nucleotide is then replaced by the repair mechanism described above. A similar mechanism functions in the repair of lesions resulting from depurination, the loss of a guanine or adenine base from DNA caused by hydrolysis of the glycosylic bond between deoxyribose and the base. Depurination occurs spontaneously and is fairly common in mammals and birds because of their warm body temperatures. The resulting abasic sites, if left unrepaired, generate mutations during DNA replication because they cannot specify the appropriate paired base.
