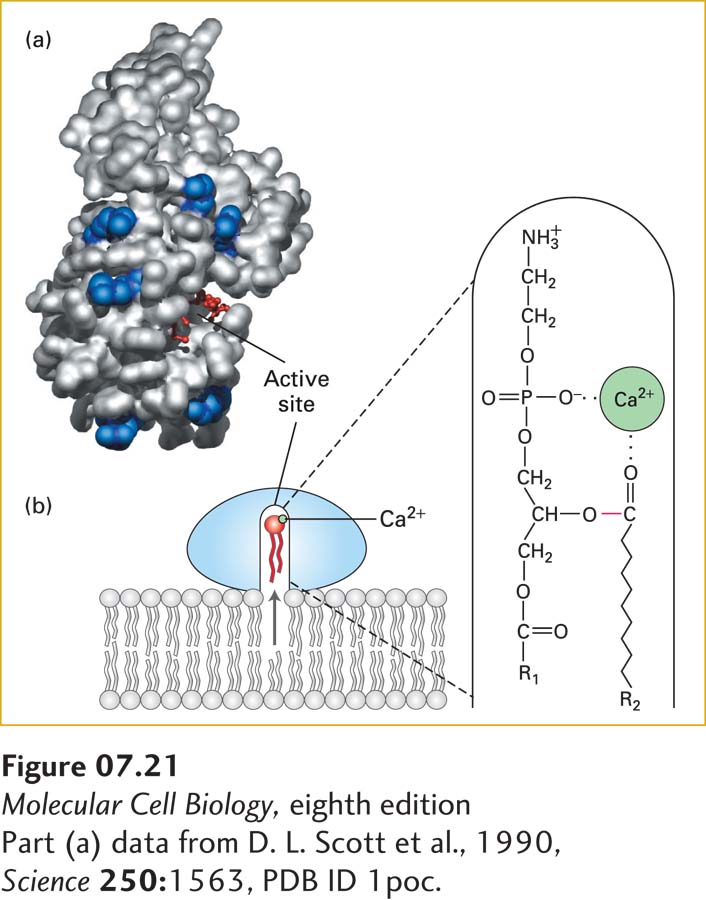
FIGURE 7-21 Lipid-binding surface and mechanism of action of phospholipase A2. (a) A structural model of the enzyme, showing the surface that interacts with a membrane. This lipid-binding surface contains a rim of positively charged arginine and lysine residues, shown in blue surrounding the cavity of the catalytic active site, in which a substrate lipid (red ball-and-stick structure) is bound. (b) Diagram of catalysis by phospholipase A2. When docked on a lipid membrane, positively charged residues of the binding site bind to negatively charged polar groups at the membrane surface. This binding triggers a small conformational change, opening a channel lined with hydrophobic amino acids that leads from the bilayer to the catalytic site. As a phospholipid moves into the channel, an enzyme-bound Ca2+ ion (green) binds to the head group, positioning the ester bond to be cleaved (red) next to the catalytic site. See D. Blow, 1991, Nature 351:444, and M. H. Gelb et al., 1999, Curr. Opin. Struct. Biol. 9:428.
[Part (a) data from D. L. Scott et al., 1990, Science 250:1563, PDB ID 1poc.]
Many water-soluble enzymes whose substrates are phospholipids must bind to membrane surfaces. Phospholipases, for example, hydrolyze various bonds in the head groups of phospholipids (see Figure 7-12) and thereby play a variety of roles in cells—helping to degrade damaged or aged cellular membranes, generating precursors to signaling molecules, and even serving as the active components in many snake venoms. Many peripheral proteins, including phospholipases, initially bind to the polar head groups of membrane phospholipids to carry out their catalytic functions. The mechanism of action of phospholipase A2 illustrates how such enzymes can reversibly interact with membranes and catalyze reactions at the interface of an aqueous solution and a lipid surface. When this enzyme is in aqueous solution, its Ca2+-containing active site is buried in a channel lined with hydrophobic amino acids. The enzyme binds with greatest affinity to bilayers composed of negatively charged phospholipids (e.g., phosphatidylserine). This observation suggests that the rim of positively charged lysine and arginine residues around the entrance to the catalytic channel is particularly important in binding (Figure 7-21a) and constitutes a lipid binding motif. Binding induces a conformational change in phospholipase A2 that strengthens its binding to the phospholipid heads and opens the hydrophobic channel. As a phospholipid molecule moves from the bilayer into the channel, the enzyme-bound Ca2+ binds to the phosphate in the head group, thereby positioning the ester bond to be cleaved in the catalytic site (Figure 7-21b), releasing the acyl chain.

