Cholesterol Is Synthesized by Enzymes in the Cytosol and ER Membrane
Next we focus on cholesterol, the principal sterol in animal cells. Cholesterol is synthesized mainly in the liver. The first steps of cholesterol synthesis (Figure 7-26)—conversion of three acetyl groups linked to CoA (acetyl CoA) forming the six-carbon molecule β-hydroxy-β-methylglutaryl linked to CoA (HMG-CoA)—take place in the cytosol. The conversion of HMG-CoA into mevalonate, the key rate-controlling step in cholesterol biosynthesis, is catalyzed by HMG-CoA reductase, an ER integral membrane protein, even though both its substrate and its product are water soluble. The water-soluble catalytic domain of HMG-CoA reductase extends into the cytosol, but its eight transmembrane α helices firmly embed the enzyme in the ER membrane. Five of the transmembrane α helices compose the so-called sterol-sensing domain and regulate enzyme stability. When levels of cholesterol in the ER membrane are high, binding of cholesterol to this domain causes the enzyme to bind to two other integral ER membrane proteins, Insig-1 and Insig-2. This binding, in turn, induces ubiquitinylation (see Figure 3-31) of HMG-CoA reductase and its degradation by the proteasome pathway, reducing the production of mevalonate, the key intermediate in cholesterol biosynthesis.
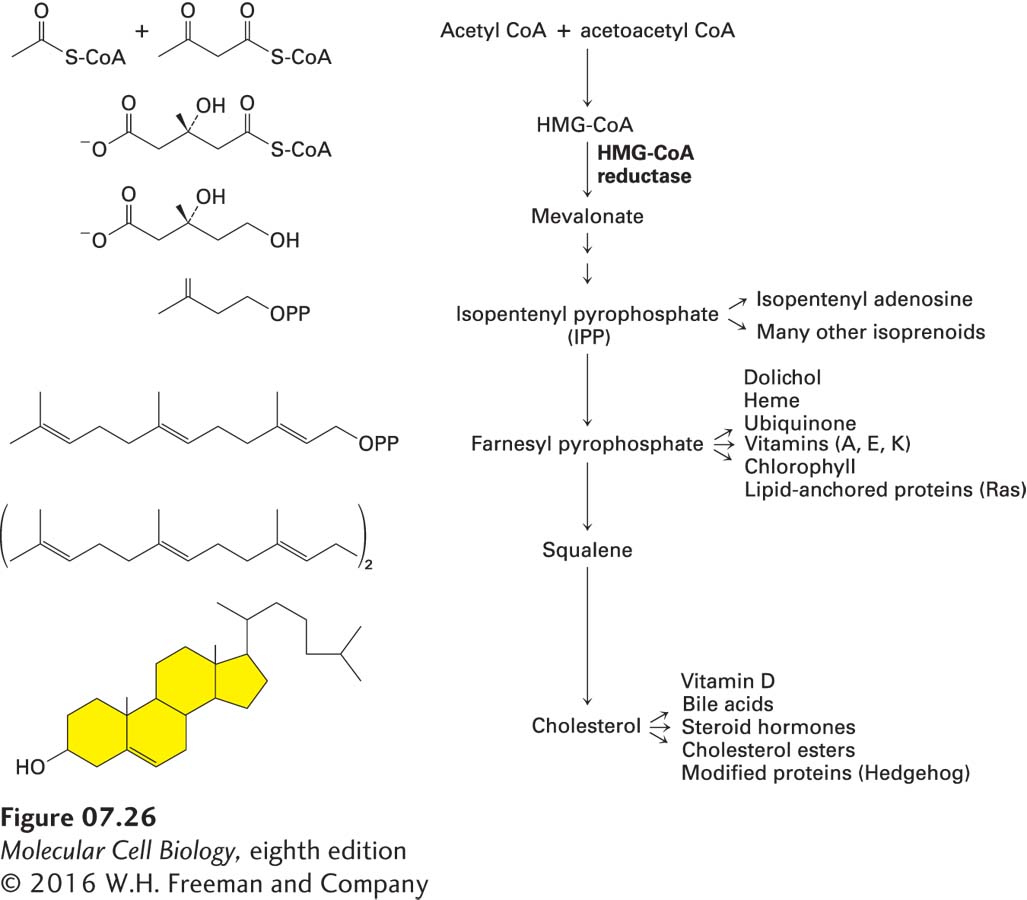
FIGURE 7-26 Cholesterol biosynthetic pathway. The regulated rate-controlling step in cholesterol biosynthesis is the conversion of β-hydroxy-β-methylglutaryl CoA (HMG-CoA) into mevalonate by HMG-CoA reductase, an ER-membrane protein. Mevalonate is then converted into isopentenyl pyrophosphate (IPP), which has the basic five-carbon isoprenoid structure. IPP can be converted into cholesterol and into many other lipids, often through the polyisoprenoid intermediates shown here. Some of the numerous compounds derived from isoprenoid intermediates and cholesterol itself are indicated.
Atherosclerosis, frequently called cholesterol-dependent clogging of the arteries, is characterized by the progressive deposition of cholesterol and other lipids, cells, and extracellular matrix material in the inner layer of the wall of an artery. The resulting distortion of the artery’s wall can lead, either alone or in combination with a blood clot, to major blockage of blood flow. Atherosclerosis accounts for 75 percent of deaths due to cardiovascular disease in the United States.
Perhaps the most successful anti-atherosclerosis medications are the statins. These drugs bind to HMG-CoA reductase and directly inhibit its activity, thereby lowering cholesterol biosynthesis. As a consequence, the concentration of low-density lipoproteins (see Figure 14-27)—the small, membrane-enveloped particles containing cholesterol esterified to fatty acids that often and rightly are called “bad cholesterol”—drops in the blood, reducing the formation of atherosclerotic plaques.
Mevalonate, the six-carbon product formed by HMG-CoA reductase, is converted in several steps into the five-carbon isoprenoid compound isopentenyl pyrophosphate (IPP) and its stereoisomer, dimethylallyl pyrophosphate (DMPP) (see Figure 7-26). These reactions are catalyzed by cytosolic enzymes, as are the subsequent reactions in the cholesterol synthesis pathway, in which six IPP units condense to yield squalene, a branched-chain 30-carbon intermediate. Enzymes bound to the ER membrane catalyze the multiple reactions that convert squalene into cholesterol in mammals or into related sterols in other species. One of the intermediates in this pathway, farnesyl pyrophosphate, is the precursor of the prenyl lipid that anchors Ras and related proteins to the cytosolic surface of the plasma membrane (see Figure 7-19) as well as other important biomolecules (see Figure 7-26).
